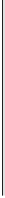Agriculture Reference
In-Depth Information
(a)
(b)
(c)
0.5
1
1.2
y
=
0
0.2
0.4
0.
0.
1
0.4
y
=
1
0
1.0
0.8
0.6
0.4
0.2
0.8
−
1
0.3
0.6
0.2
−
2
0.4
0.1
−
3
0.2
0.0
0.0
−
4
0.0
0.4
0.8
1.2
1.6
0.0
0.4
0.8
1.2
1.6
0.0
0.4
0.8
1.2
1.6
CH
4
produced/CO
2
produced (
f
)
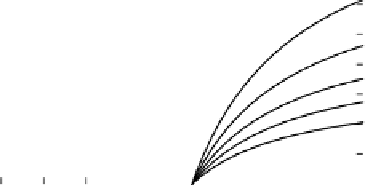
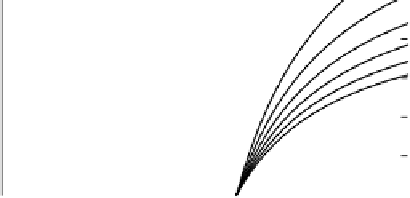
Figure 5.4
Calculated (a) electron balance (Equation 5.5), (b) production of acetate
(Equation 5.6) and (c) production of H
2
(Equation 5.7) as functions of the ratio of CH
4
to CO
2
produced
(φ)
during anaerobic decomposition of soil organic matter. Numbers on
curves are ratios of CH
4
produced from H
2
+
CO
2
to CH
4
produced from acetate
(ψ)
These relations are plotted in Figure 5.4(b) and (c), showing how the amounts
of H
2
and acetate produced per mole of C consumed vary with the ratio of CH
4
to CO
2
produced.
Finally, from Equation (5.4) the rate of change in
Z
is
d
Z/
d
t
=
(
2
c
−
4
d)V
1
=
2
V
H
2
−
4
(V
CO
2
−
V
Ac
+
V
H
2
/
4
)
(
5
.
8
)
where
V
Ac
=
(
1
−
R
H
2
)V
CH
4
and
V
H
2
=
4
R
H
2
V
CH
4
, giving
d
Z/
d
t
=
4
(V
CH
4
−
V
CO
2
)
(
5
.
9
)
Yao and Conrad (2000) measured rates of CO
2
and CH
4
production (
V
CH
4
and
V
CO
2
) and the proportion of CH
4
produced from H
2
+
CO
2
(R
H
2
)
in a range of
submerged rice soils under pseudo steady-state conditions, and calculated the
electron balance using Equation (5.9). The results are shown in Figure 5.5. The
figure shows that in the majority of the soils there was a net deficit of electrons,
indicating that the SOM became more reduced during decomposition. The ratio
φ
=
V
CH
4
/V
CO
2
varied from 0.39 to 0.96 in six of the soils, and from 1.24 to 1.36
in the remaining two. Also a fairly small proportion of the CH
4
was produced
from reduction of CO
2
with H
2
; most was produced from disproportionation of
acetate. The ratio
ψ
=
R
H
2
/R
Ac
varied from 0.27 to 0.54.
There are various possible explanations for the decrease in oxidation state of
the SOM carbon in most of the soils. One is that the SOM comprises different
pools of organic matter with carbon in different oxidation states, and
Z
decreases
as a result of preferential oxidation of more-oxidized pools of SOM, leaving a
greater proportion of the more-reduced forms in the residue. The more oxidized
SOM would include compounds in the original SOM and also compounds gen-
erated in the course of reduction, for example as a result of chemical oxidation
by Fe(III) and other metal oxides. Alternatively, part of the organic matter could