Agriculture Reference
In-Depth Information
(a)
net current
(b)
−
100
−
100
Fe
3
+
→
Fe
2
+
−
i
0
50
−
50
−
Fe
3
+
→
Fe
2
+
←
Potential (mV)
net current
0
0
525
500
475
525
475
Fe
2
+
→
Fe
3
+
50
+
i
0
50
Fe
2
+
→
Fe
3
+
100
100
(c)
−
50
net current
Fe
3
+
→
Fe
2
+
−
i
0
0
525
500
475
4
50
+
i
0
425
+
i
0
50
Fe
2
+
→
Fe
3
+
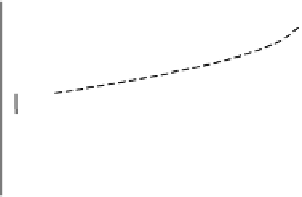
Electrode current versus electrode potential curves for the Fe
2
+
-Fe
3
+
couple
Figure 4.8
(a) [Fe
3
+
]
=
[Fe
2
+
]
=
1mM; (b) [Fe
3
+
]
=
[Fe
2
+
]
=
0
.
1mM;
in
water
at
pH
2
with
(c) [Fe
3
+
]
0
.
1mM, [Fe
2
+
]
1cm
2
=
=
1 mM.
Electrode
area
=
(Stumm
and
Morgan,
1996). Reproduced by permission of Wiley, New York
for [Fe
3
+
]
=
[Fe
3
+
]
=
10
−
3
M (Figure 4.8a). If the concentration of both ions
is 10-fold smaller,
i
0
and the slope are 10-fold smaller (Figure 4.8b). How-
ever if the concentration of only one of the ions is decreased the drop in
i
0
is not as great (Figure 4.8c); note also that the equilibrium potential is shifted.
If [Fe
3
+
]
=
[Fe
3
+
]
=
10
−
7
M,
i
0
≈
0
.
1
µ
A and measurements are no longer reli-
able. In practice the limiting concentration is nearer 10
−
5
M because of the effects
of trace impurities. The value of
i
0
will increase with the surface area of the
electrode. However the benefit of this tends to be offset by greater effects of
impurities. In the case of the O
2
-H
2
O couple, the net current is virtually zero
over a wide range of electrode potentials as shown in Figure 4.9(a). This makes it
extremely difficult to determine the equilibrium potential for the O
2
-H
2
O couple,
and so
E
H
measurements in aerated soils are not reliable.
A further problem, particularly in soil systems, is that several redox systems
may be present, in which case the apparent equilibrium potential may be the result