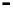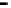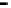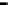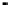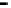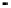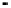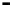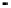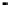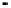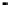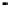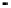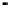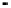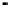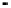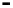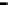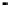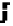Agriculture Reference
In-Depth Information
(a)
(b)
H
2
O
→
H
2
−
1
O
2
→
H
2
O
O
2
→
H
2
O
Fe
3
+
→
Fe
2
+
0
0
+
1
0
−
1
E
m
E
eq
Potential (V)
Potential (V)
H
2
O
→
O
2
Fe
2
+
→
Fe
3
+
1
Figure 4.9
Electrode current versus electrode potential curves for solutions containing
O
2
: (a) in otherwise pure water; (b) in the presence of Fe
2
+
. In (a) the net current is
close to zero over a wide range of potential, so it is difficult to locate the equilibrium
potential. In (b) the measured equilibrium potential is a mixed potential,
E
m
, obscuring
the true equilibrium potential of the system,
E
eq
(Stumm and Morgan, 1996). Reproduced
by permission of Wiley, New York
of the combined exchange currents of two or more redox couples. Figure 4.9(b)
illustrates this for the Fe
2
+
-Fe
3
+
system in the presence of trace concentrations
of dissolved O
2
. The measured equilibrium potential,
E
m
, at which the net current
is zero may be the potential at which the rate of reduction of O
2
at the electrode
equals the rate of Fe
2
+
oxidation. This would be likely if the concentration of
Fe
2
+
greatly exceeded that of Fe
3
+
, as in general it will in submerged soils. The
two couples are not in equilibrium with each other and the measured potential
is termed a mixed potential. The mixed potential does not represent either of the
individual couples operating and is therefore difficult to interpret. Many redox
couples do not react reversibly at electrode surfaces. Examples are CO
2
-CH
4
and NO
3
−
-N
2
. This too complicates interpretation.
These factors rather constrain the usefulness of
E
H
measurements in soil solu-
tions. Inferences about the thermodynamics of redox processes in soils that rely
heavily on measurements of redox potential should be treated with caution.
Nonetheless soil
E
H
measurements provide a ready measure of redox status,
for example in experiments in which constant
E
H
and pH are required (Patrick
et al
., 1973).
4.3 TRANSFORMATIONS OF NUTRIENT ELEMENTS
ACCOMPANYING CHANGES IN REDOX
These are briefly discussed here in the context of redox chemistry. More complete
discussions are given in Chapters 5 - 8.