Chemistry Reference
In-Depth Information
N
N
N
N
*
Ag,
exch
L,L
G
+ 2
+ 2
N
N
N
N
N
R
h
= 6.9Å
R
h
= 7.5Å
N
N
N
[Ag
2
L
2
]
2+
L
*
[Ag
2
L
*
2
]
2+
L
2
N
e
1
Ag,Ag
Av
E
Coulomb:
+100
+76
gas
4
a
0
2
N
e
1
4
Born:
-391
0
-360
Av
G
1
solv
8
R
0
solv
h
Balance: -291
-284
Ag,Ag
Ag,Ag
0
0
E
E
284
291
2
G
G
*
*
L
,sol
solv
L
solv
L
,sol
L
Figure 3.19 Quantitative analysis of the Coulomb and Born energetic contributions to the
apparent intermetallic interactions in solution. (
a
is the intermetallic separation, R
h
is the
pseudo-spherical hydrodynamic radius.
[13] Reproduced by permission of The Royal Society
of Chemistry.
parameters remain unchanged [19]. Although the variations of Coulomb and Born ener-
getic contributions are relatively important, the overall balance is only few kJ/mol. More-
over, the decrease of intermetallic distance with the concomitant increase of Coulombic
repulsions contra-intuitively produces a more favourable solvation effect, which appar-
ently induces positive intermetallic interactions. Therefore, it is not so surprising to exper-
imentally find positive or negative values of homocomponent interaction energies in
solution [19,49].
In summary, an efficient and thought-out manipulation of subtle homocomponent inter-
actions (cation-cation, ligand-ligand) is challenging. Moreover, these parameters may be
strongly sensitive to minor structural changes in the supramolecular structure. The com-
pensation of Coulombic interaction by solvation energies must be taken into account in
the predictive design of stabilizing interactions in self-assembly. In this context, a favour-
able solvation effect may be responsible for the stabilization of compact polynuclear com-
plexes (i.e., tetranuclear 3D structures [22]) despite a large number of intermetallic
repulsive interactions. Alternatively, the use of negatively charged binding units instead
of neutral ones maximizes the coordination interactions, and the charge neutralization
contributes to a decrease of intermetallic repulsions, which considerably favours the ulti-
mate energetic balance [15a,39].






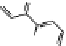


























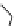


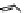
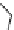

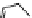










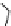








Search WWH ::

Custom Search