Chemistry Reference
In-Depth Information
Structures
Point groups
Microconstants
M,
L
12
k
M
C
s
1,1
2
6
M,L
M
L
,
L
M
,
M
C
2v
36
k
u
u
2,1
1
2
1
2
2
M,L
1,2
M
L
,
L
C
1
48
k
u
12
4
2
C
2
M,L
144
k
M
u
L,L
u
M,M
EM
2,2
1
2
1
2
3
3
C
3
M,L
32
k
M
u
L
,
L
1,3
1 2
6
6
2
D
3
M,L
96
k
M
u
L,L
u
M,M
EM
2,3
1
2
1
2
Figure 3.16 Schematic structure, symmetries and statistical factors for all possible microspe-
cies formed between L and metal ions, including the dinuclear triple-stranded helicate [M
2
L
3
]
[15c].
law and (iii) the effective concentration of long-distance macrocyclization processes is
estimated with statistical theory [23]. Finally, the system of complexes was reasonably
described with five thermodynamic parameters, which were extracted by nonlinear fitting
procedures [15c]. The free energy of intermolecular connections for two different binding
sites in the analysed system (D
G
E
N
3
¼
-31(1) kJ/mol, D
G
E
N
2
O
¼
-33(1) kJ/mol) are very
similar. The effective molarity term decreases the energetic gain of each intramolecular
reaction by D
G
Eu
corr
6(5) kJ/mol. Despite this, the inter- and intramolecular connections
represent the most favourable energetic contributions to the total free energy of formation.
In contrast, the homocomponent interaction parameters are positive (D
E
Eu;Eu
12
¼
¼
10(4) kJ/
mol, D
E
L;L
12
6(2) kJ/mol) and thus disfavourable for complex formation. Both factors
contribute to the overall negative allosteric chelate cooperativity, which may be expressed
as the coefficient a in Equation 3.14 [11,15a].
¼
Y
Y
D
E
M;M
k
D
E
L;L
l
a ¼
exp
=
RT
exp
=
RT
ð
3
:
14
Þ
k
l
An even more complex package of
d
-and
f
-heteronuclear helicates was analysed by
Riis-Johanessen
et al.
[49]. The complex fitting procedure allowed the direct determina-
tion of apparent long-range intermetallic interactions without making any restrictive
assumptions. The obtained results demonstrate a contra-intuitive alternation of attractive
and repulsive interactions with the intermetallic distance, which is explained by the
changes in solvations energies with respect to geometrical parameters.
Thermodynamic modelling with TAFEM was also successfully extended to 3D heli-
cates formed with tripodal ligands and lanthanides [22]. The fitted parameters are remi-
niscent of the values previously obtained for the system of different linear triple-stranded



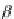
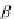

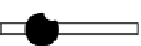























































































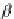








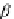







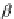




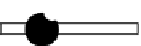


















































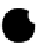


























































Search WWH ::

Custom Search