Chemistry Reference
In-Depth Information
N
Eu
N
3
N
k
N
O
N
Eu
N2O
k
N
N
N
N
N
3
N
N
N
N
N
N
N
N
O
N
+ 4
M
3+
6
6
12
4.33
Eu
Eu
6
Eu,L
L,L
Eu,Eu
3456
k
k
u
u
EM
1-2
1-2
4,3
N
2
O
N
3
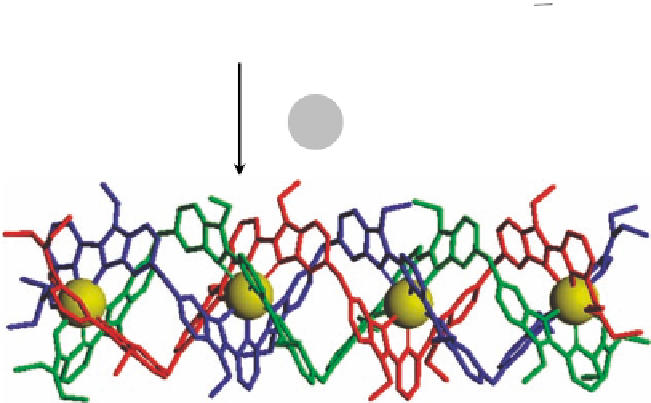
Figure 3.17 Prediction of tetranuclear triple-stranded helicate with TAFEM [15c,51].
helicates [15c]; however, a limited set of stability constants compromises a more sophisti-
cated interpretation of data. Analogously, more reliable parameters can be obtained by
fitting a larger set of thermodynamic data eventually collected for tetranuclear and penta-
nuclear 3D complexes with helicate segments [40].
The first predictive application of TAFEM resulted in an estimation of the thermo-
dynamic stability constant of a tetranuclear europium triple-stranded helicate (Figure
3.17). Using the microscopic parameters extracted from the set of dinuclear and trinuclear
helicates, the calculated stability constant amounts to log
Eu
;
L
5[50] and closely
agrees with the value determined using spectrophotometric titrations log
b
¼
42
:
4;3
Eu
;
L
b
¼
4;3
43
[15c]. A calculation of system speciation with TAFEM also proved to be effi-
cient for a non-helical system consisting of Pd(II) complexes with a bipy ligand. A knowl-
edge of microscopic affinity constants allowed the construction of speciation maps for
different concentrations and a prediction of the best conditions for the assembly of molec-
ular squares [51].
:
2
ð
1
:
9
Þ
3.5.3 Solvation Energies and Electrostatic Interactions
The cooperativity factors in supramolecular compounds, accessed for instance through
thermodynamic modelling (Equation 3.14), are usually interpreted in terms of electro-
static interactions, steric hindrance and so on. However, the correct estimation of the free
energy contribution in solution requires a consideration of solvation energies that








































































Search WWH ::

Custom Search