Chemistry Reference
In-Depth Information
Excess of L
R
N
+ Eu
+
L
N
N
N
[Eu
L
]
3+
[Eu
L
2
]
3+
[Eu
L
3
]
3+
N
+ Eu
L
+
L
N
N
N
[Eu
2
L
]
6+
[Eu
2
L
2
]
6+
[Eu
2
L
3
]
6+
N
N
Excess of Eu
3+
R
(a)
Excess of L
OH
+ Eu
+
L
O
N
N
[Eu(
L
-2H)]
+
[Eu(
L
-2H)
2
]
-
N
+ Eu
+ Eu
L
+
L
+
L
N
N
N
O
[Eu(
L
-2H)]
4+
[Eu
2
(
L
-2H)
2
]
2+
[Eu
2
(
L
-2H)
3
]
0
OH
Excess of Eu
3+
(b)
Figure 3.11 Schematic representation of the self-assembly mechanisms for triple-stranded
lanthanide helicates [Eu
2
L
3
].
(a) Adapted with permission from [38b]. Copyright 2003 Ameri-
can Chemical Society. (b) Adapted with permission from [ref 39b] Copyright 2011 WILEY-
VCH Verlag GmbH & Co. KGaA, Weinheim.
be a key reaction intermediate in the formation of triple-stranded helicates. While [Eu
2
L
2
]
with a neutral ligand is a labile “side by side” species, the analogous complex with a
charged ligand apparently adopts a helical form, which is already well preorganized for
braiding of the third ligand. This terminal step clearly occurs with positive cooperativity,
and probably all kinds of cooperative interactions (see Section 3.3) contribute to the ther-
modynamic stabilization of the final triple-stranded helicate (Figure 3.11b).
All kinetic studies showed a stepwise formation of helicates and supramolecular com-
pounds, which definitively disproves the hypothesis of “magic” self-assembly processes
and confirms the validity of classical coordination chemistry. It has been shown that the
self-assembly mechanism and the reaction rates significantly depend on stoichiometric
conditions and on the magnitude of electrostatic interactions. A final slow rearrangement
step is often observed and applies to self-repair and thermodynamic re-equilibration. This







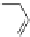
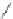



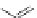



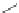






































































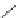

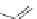







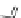

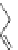

































































Search WWH ::

Custom Search