Chemistry Reference
In-Depth Information
X
X
N
N
+ 2 Cu
+ L
O
X
[Cu
2
L]
2+
[Cu
2
L
2
]
2+
N
+ Cu
N
X
+
O
N
[Cu
2
L
2
]
3+
[Cu
3
L
2
]
3+
N
X
X
[Cu
3
L
2
]
3+
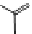
Figure 3.9 Schematic representation of the self-assembly mechanism for the double-stranded
helicates [Cu
3
L
2
]
3þ
.
Adapted with permission from [36]. Copyright 2001 Wiley-VCH Verlag
GmbH & Co. KGaA.
of two metal ions to the same ligand does not occur because the intermetallic repulsions
are apparently too strong. However, using a negatively charged analogous ligand, the
metal charges are neutralized and helicates are observed even in metal excess (Figure
3.11b). In both systems, the conditions in ligand excess are unfavourable because of
strong negative interligand interactions (steric hindrance, charge repulsion). Likewise, the
reaction propagates through the dinuclear unsaturated species [Eu
2
L
2
], which appears to
Excess of L
L
[Fe
L
2
]
2+
o
-[Fe
L
2
]
2+
[Fe
L
3
]
2+
[Fe
2
L
3
]
4+
s
s
s
s
s
+ Fe
2+
+ Fe
2+
N
s
N
O
NH
Excess of Fe
2+
HN
O
+ Fe
2+
s
s
s
N
N
s
[Fe
L
2
]
2+
[Fe
2
L
2
]
4+
Figure 3.10 Self-assembly mechanism for the formation of diferrous triple-stranded helicates
[Fe
2
L
3
]
4þ
(S¼ solvent molecules).
Reprinted from [14] with permission from Elsevier.



























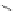


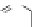


















































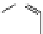






































































Search WWH ::

Custom Search