Biomedical Engineering Reference
In-Depth Information
on the hydroxyapatite structure, in order to comprehend where and how this ion is located.
The results of these studies assert that the CO
3
2-
can substitute an OH
-
ion of the structure, a
type A
defect, or a PO
4
3-
, a
type B
defect. This hypothesis has been confirmed by IR spectra in
which the vibrational mode frequencies of the carbonate are different for each type of
substitution.
As the net charge of the carbonate is different from those of the substitutional anions, charge
compensation is required.
In the case of the type A defect, the charge excess can be compensated by creation of an ionic
vacancy removing another OH ion, also because of the larger steric encumbrance of the
carbonate ion with respect to the hydroxyl ion (Peroos et al., 2006).
In the case of the type B defect, the electro-neutrality can be maintained in five different
ways (Astala & Stott, 2005):
1.
Removal of one OH ion and creation of one Ca vacancy (B1 complex);
2.
Removal of one Ca ion for every two carbonate ions (B2 complex);
3.
Substitution of one Ca with one hydrogen (B3 complex);
4.
Substitution of one Ca with one alkaline ion;
5.
OH ion incorporation close to the carbonate ion.
When considering the type A defect, it is first necessary to notice that, in the hydroxyapatite
unit cell, only two OH ions are present (Fig. 1). If they are both removed at once but only
one carbonate ion is included, the resulting structure is no longer a hydroxyapatite, as it had
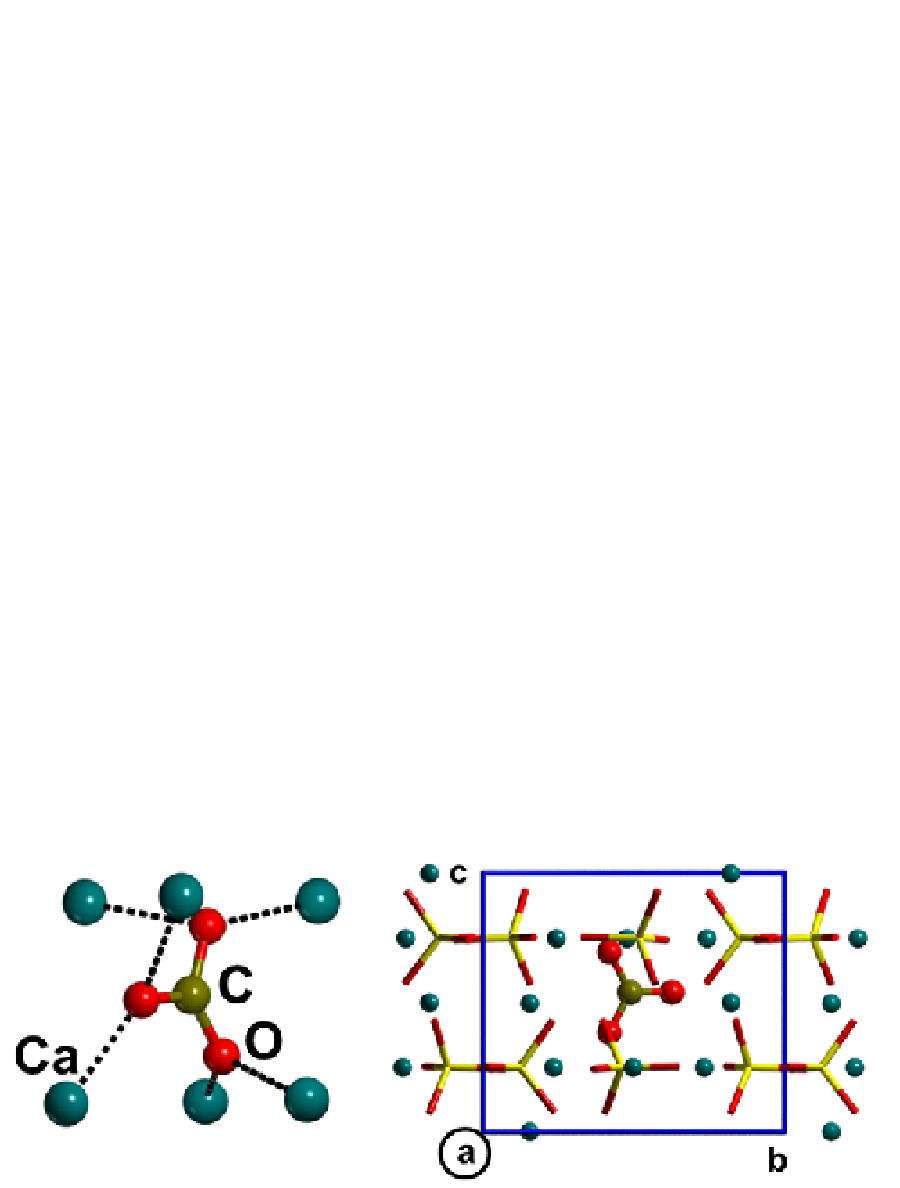
lost all the hydroxyl ions, and has to be considered a carbonated apatite. In Fig. 5, this stable
structure is reported. The similarity between carbonate apatite and calcite structures is clear:
the averaged distance <Ca-OC> is 2.35 Å in the carbonate apatite and 2.34 Å in the calcite.
From the
ab initio
calculation, it is also possible to predict the enthalpy of formation of the
carbonate apatite from the calcium phosphate and the calcite structures, considering the
reaction (2):
3Ca
3
(PO
4
)
2
+ CaCO
3
→ Ca
10
(PO
4
)
6
(CO
3
)
(2)
The B3LYP enthalpy of formation is -35 kJ/mol, directly comparable with the value of -32
kJ/mol obtained with the VASP code and the PW91 functional (Rabone & de Leeuw, 2007).
Fig. 5. Carbonated apatite. On the left side, a view of the carbonate ion is reported,
highlighting its distances to the closest Ca ions. On the right side, the cell, where both OH
are removed and one carbonate is substituted, is reported after full relaxation of the atoms.