Biomedical Engineering Reference
In-Depth Information
clear whether this is due to the multiple carboxyl groups of this salt, to its strong metal ion
chelating capability, or to some other property. However, certain other marine adhesives,
such as that from
Mytilus
, do require metal activity (Hwang et al., 2010). A previous study
which tested 15 different amino acids at 0.5% w/v solutions on adhesion by
H. forskåli
tubules (Müller et al., 1972) showed that most had little, if any, effect. The exceptions were
the hydrophobic amino acids leucine (20% loss) and phenylalanine (57% loss). For
phenylalanine, the loss was slow to develop (taking several minutes) and could not be
reversed by washing (Zahn et al., 1973).
Solution
Force/width
S.D.
n
(N/mm)
3.5% NaCl > 0.050 >8
Tris/chloride 0.050 0.008 6
Sodium formate 0.047 0.012 6
Sodium oxalate 0.046 0.011 8
Ammonium chloride 0.036 0.008 8
Sodium citrate 0.035 0.009 7
Sodium acetate 0.030 0.006 8
Sodium EDTA <0.003 8
Table 3.
Comparison of the effects of different salt solutions on adhesion of Cuvierian
tubules onto glass. All salts were 50mM in 3.5% NaCl, 10 mM sodium phosphate, pH 7.6
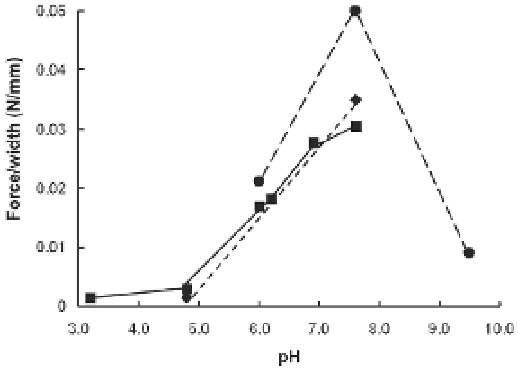
The effect of pH on the adhesive strength of the
H. dofleinii
adhesive showed that for
Tris/chloride buffer, the best observed strength of those tested was at pH 7.6, and that the
observed strength decreased at both lower and higher pH values (Figure 6). For citrate and
acetate buffers, adhesive strength declined progressively as the pH was lowered from pH
7.6, with little adhesion remaining at pH 5.0 (Figure 6). A loss of adhesive strength at acidic
pH values was also observed by Müller et al. (1972), who used paraffin wax as a (poor)
substratum for tubule adhesion.
Fig. 6. The effect of different washing solutions on the adhesiveness of
H. dofleinii
Cuvierian
tubules for glass. The effect of changes in pH, where ■ indicates acetate buffers, ♦ indicates
citrate buffers and ● indicates Tris/Chloride buffers.