Biomedical Engineering Reference
In-Depth Information
(N/mm)
Glass > 0.050 - 10
Aluminium > 0.050 - 6
Polyvinyl chloride 0.024 0.006 11
Chitin 0.021 0.005 8
Polycarbonate 0.010 0.002 8
PMMA 0.009 0.003 7
PTFE 0.008 0.002 6
Table 2. Force required to peel Cuvierian tubules off various substrata to determine
adhesive strength.
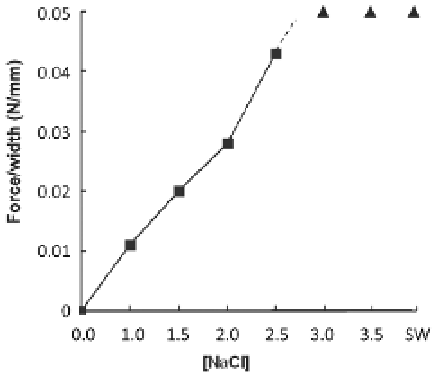
Fig. 5. The effect of different washing solutions on the adhesiveness of
H. dofleinii
Cuvierian
tubules for glass. The effect of NaCl concentration; where ▲ indicates conditions where the
force per unit width exceeded 0.05 N/mm. SW is natural sea water.
Adhesive strength decreased with decreasing NaCl concentration (Figure 5). At ≥3% NaCl
the adhesion exceeded 0.05 N/mm. Reducing the NaCl concentration incrementally from
2.5% to 1.0% NaCl led to a steady decline in adhesive strength (Figure 5). The adhesive
strength at 1% NaCl, which is comparable in concentration to physiological saline, was
significantly weaker than in 3.5% NaCl simulated seawater. This is consistent with the
previous observations on
H. forskåli
tubules (Flammang et al., 2002). It suggests that
hydrophobic interactions may be important in the adhesive mechanism.
The effects on tubule-glass adhesion of other chloride or sodium salts (50 mM) (Table 3)
showed that in all cases there was a loss of adhesive strength. For chloride salts, the loss was
smaller when Tris rather than ammonium was the cation (Table 3). The other salts examined
were all sodium salts of carboxylic acids, for whose action no simple mechanism could be
proposed. Thus while formate (a monocarboxylate) and oxalate (a dicarboxylate) both
showed similar adhesion, that observed with acetate (another monocaboxylate) was ~35%
below the value observed for formate. However, the values presented in Table 3 show only
a trend as the errors in measurement are such that the different systems are not necessarily
distinguishable. Supplementation with EDTA (a tetracarboxylate) was the most effective at
disrupting bond strength, and essentially led to complete loss of adhesion (Table 3). It is not