Biomedical Engineering Reference
In-Depth Information
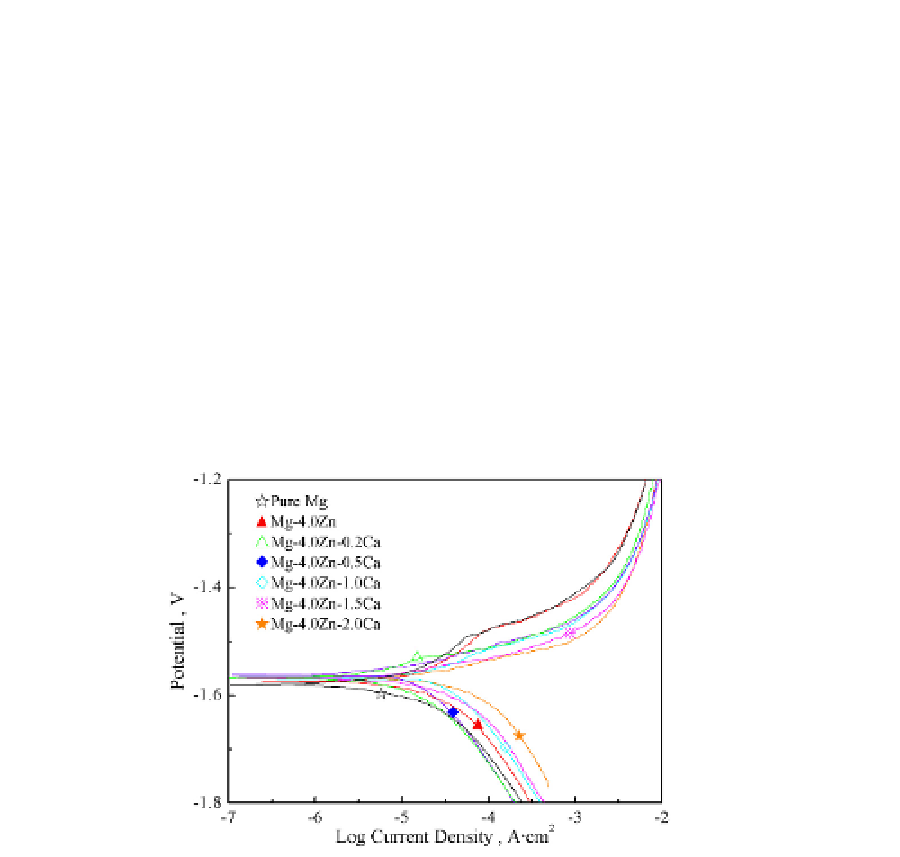
xCa alloys are enhanced to -1574mV, which is increased by 70 mV compared with -1646 mV
of the pure Mg corrosion potential. However, it is confirmed that Mg-4.0 Zn-0.2 Ca alloy
exhibits the best corrosion resistance among Mg-4.0Zn-xCa alloys, even higher than that of
Mg-4.0 Zn alloy through further observation. This particular phenomenon can be explained
as follows. Firstly, the addition of 4.0 wt. % Zn can cause the formation of coarse MgZn
precipitate as shown in Fig.4 and 2(a), which reduces the corrosion resistance of Mg-4.0Zn
alloy due to the different electrochemical behaviors between primary α-Mg and precipitate.
Then, the slight addition (less than 0.5 wt. %) of Ca alloying element can cause MgZn
precipitates to be effectively transform to fine ternary precipitates, which has been clearly
documented in previous literature [27][28]. The refinement and homogenization of
precipitate phase can improve the corrosion resistance of Mg-4.0Zn-0.2Ca alloy compared
with that of Mg-4.0Zn alloy. Finally, increasing Ca content was over than 0.5wt. % cause the
formation of another coarse Mg
2
Ca, Ca
2
Mg
6
Zn
3
and Ca
2
Mg
5
Zn
13
precipitates as shown in
Fig.3 and 4. It is quite obvious that the precipitates increases with Ca content increasing,
which decreases the corrosion resistance of as-cast Mg-4.0Zn-xCa alloys.
Fig. 9. The potentiodynamic polarization curves of as-cast Mg-4.0 Zn-x Ca alloys in Hank's
solution.
It is well known that the cathodic polarization curves represent the cathodic hydrogen
evolution through water reduction, while the anodic polarization curves do the dissolution
of magnesium. That is to say, it is equivalent to that Mg-4.0 Zn-0.2 Ca alloy sample exhibits
the lowest current of hydrogen evolution reaction and Mg-4.0 Zn-1.5 Ca and Mg-4.0Zn-2.0
Ca alloys samples does the highest ones, which indicates that over potential of the cathodic
hydrogen evolution reaction of Mg-4.0 Zn-0.2 Ca alloy is much lower than those of Mg-4.0
Zn-1.5 Ca and Mg-4.0 Zn-2.0 Ca alloys. Therefore, the lowest cathodic hydrogen evolution
reaction brings the highest corrosion resistance to the Mg-4.0 Zn-0.2 Ca alloy.
Fig.10 illustrates the pH variation of Hank's solution versus the immersion testing time for
Mg-4.0Zn-xCa alloys. It could be observed that the pH variations of the alloys all obey the
parabolic rate law. The pH variation rate decreases with the immersion time increasing.
After 48 hrs immersion, all the pH values of the samples tend to be stable. In the early
period of immersion, both pure Mg and the Mg-4.0Zn-xCa alloys acutely reacted with