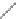Environmental Engineering Reference
In-Depth Information
O
R'
+HB
-B
-
R'CHNO
2
+R-CHO
R'CH
2
NO
2
RCH
CH
NO
2
RCHCH
NO
2
OH
R'
Acetal from the formed multi-nitro alcohol is the main energetic ingredient in
liquid mixed explosives [
19
], and can be used as a rocket propellant. Formaldehyde
and 2,2-dinitropropanol can generate acetal through addition reaction. The con-
densation reaction can be catalyzed by 96 % H
2
SO
4
, calcium chloride, zinc chlo-
ride, ferric chloride or boron tri
uoride via catalytic polycondensation.
Mannich condensation reaction can occur between nitroalkanes and aldehydes
with amines.
fl
t-nitroalkane does not have
α
-hydrogen atoms and can not have the
Mannich condensation reaction.
R
R
C
H +HCHO
+
HN
C
CH
2
N
+H
2
O
R'
R'
-Hydrogen atoms in primary and secondary nitroalkanes are acidic, can make
this reaction smoothly to generate an nitroalkylamine. A plurality of intermediates
and many explosives can be synthesized through this reaction. Tertiary nitro
compounds or tertiary amine can not have Mannich condensation reaction. In
Mannich condensation reaction, in addition to formaldehyde, acetaldehyde and
propionaldehyde also can be used.
Nitroalkane also can reacts with carbon
α
carbon double bonds via Michael
nucleophilic addition reaction. And nitroalkanes with
-
α
-hydrogen atom can have
such reaction under alkaline catalytic conditions.
CH
3
NO
2
CH
3
CH
3
2
CCHA
Alk
a
li
H
3
C
C
+H
NO
2
C
CH
2
CH
2
A
CH
3
A in the equation represents a substituted aldehyde. An electron withdrawing
group can activate the double bond and its the electron-withdrawing capability is
related to the produced electronegativity, which are usually believed that nitro is the
strongest followed by sulfonic acid group [
20
]. The double bond is between the
substituted
-Unsaturated carboxylic acids, carboxylic acid esters,
nitriles, acids, ketones, sulfones, aldehydes, ethers, ole
α
,
ʲ
carbons.
α
,
ʲ
ns and heterocyclic alkene
can all react with nitroalkane via addition reactions to form the corresponding nitro-
derivatives.
-NO
2
>-SO
3
R>-CN>-COOR>-CHO>-COR