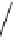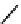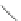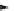Environmental Engineering Reference
In-Depth Information
H
3
C
CH
3
4CH
3
CH
2
NO
2
+CH
3
CN+2HNO
2
+3H
2
O
H
3
C
N
O
Primary and secondary nitroalkanes can react with sodium alcohol to form the
adducts.
O
O
R
R
+R''ONa
R'
N
N
ONa
CH
R'
CH
O
OR''
Dinitro compounds can be generated from slats of primary and secondary ni-
troalkanes, and secondary nitroalkane through
-halogenation, electrochemical
reaction, oxidation or automatic decomposition [
13
α
16
].
(4) Reaction of an Oxidizing Agent and a Reducing Agent
Primary nitroalkanes react with oxidants to generate aldehydes, while ketones
are generated from secondary nitroalkanes and oxidants. And this reaction can
occur on nitro groups of aromatic ring [
17
].
-
O
+O
2
(H)R'
(
H)R'
C
N
O
+NaNO
3
C
R
R
ONa
Nitrobenzene and nitro group on a aromatic ring can be reduced into hydrox-
ylamine, oxime or amine. Only with a protective agent, it can be reduced nitroso
compound, which is an intermediate reaction. If it starts from primary or secondary
nitro compounds, oxime will be formed quickly through molecular rearrangement.
When there is
-hydrogen atom, nitroso compounds can be obtained. When the
reducing ability of agent is not enough, the product is a carbonyl compound, which
will be rearranged into an oxime and further hydrolyzed into the corresponding
aldehyde or ketone in acidic medium [
18
].
(5) Addition Reaction
Henry reaction can occur between nitroalkane and aldehyde to form
α
-nitroal-
cohol. Lower nitroalkanes can react with formaldehyde through addition reaction to
form
ʲ
ʲ
-nitroalcohol.
NO
2
NO
2
OH
R
C
H + R''CHO
RC
R'
C
H
R''
R'
first step of this reaction under basic catalysis is addition reaction of an
nucleophilic reagent with a carbonyl group as the following:
The