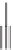Chemistry Reference
In-Depth Information
based on catalytic enantioselective cyclopropenation followed by an intramolecular PKR
established the quaternary center. The transformation of cyclopropene
84
into tricyclic PK
product
85
failed when N-oxides were used as promoters. Optimal reactivity was achieved
using tetramethylthiourea (TMU); this promoter was found to be very effective even with
substoichiometric amounts of Co
2
(CO)
8
(Scheme 4.22).
EtO
2
C
EtO
2
C
EtO
2
C
TMS
TMS
TMTU
Me
Me
Me
Me
Me
+
TMS
O
O
Co
2
(CO)
8
(60 mol %)
Me
SiMe
2
Ph
SiMe
2
Ph
SiMe
2
Ph
84
85
(65 %)
86
(16 %)
EtO
2
C
TMS
Me
Me
Me
Me
O
Me
Me
SiMe
2
Ph
H
(-)-
pentalenene (87)
85
Scheme 4.22
An intramolecular Pauson-Khand reaction used to establish the quaternary
center.
In the same year, the diastereoselective synthesis of
91
, which is a key intermediate
of naturally occurring anti-HIV isolitseane B and its analogs, via diastereoselective PKR
was reported by Coquerel and Rodriguez
et al
.
25
In this work, the introduction of a sulfur
atom not only improved the yield and stereoselectivity of the PKR but also enabled the
isolation of the final product
91
, in an enantiopure form by diastereomeric separation of an
intermediate bearing a chiral, configurationally defined, sulfur atom (Scheme 4.23).
OEt
O
O
O
OH
PhS
PhS
Co
2
(CO)
8
H
OEt
H
H
OEt
Toluene, rt, 15 min;
reflux 1.5 h
O
O
SPh
88
89
90
91
Yield 69 %
dr = 6:1
Scheme 4.23
Diastereoselective synthesis of a key intermediate
91
of anti-HIV Isolitseane B
and its analogs.
In 2007, an interesting study on the intramolecular PKR of 1,8-enynes derived from
salicylaldehyde derivatives was reported by Lovely
et al
.
26
Substrates derived from salicy-
laldehyde
92
itself reacted poorly in this reaction but related substrates containing
ortho
-
tert
-butyl substituents reacted effectively: in many cases the cyclizations proceeded with
high levels of diastereoselectivity (dr up to 10:1) and good yields (up to 95%) (Scheme 4.24).