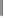Chemistry Reference
In-Depth Information
SiMe
3
SiMe
3
SiMe
3
O
O
O
CH
2
CH
2
OBzl
OBzl
OSi-
t
BuMe
2
OBzl
OSi-
t
BuMe
2
OR
6a
(R = H)
6b
(R = Si-
t
BuMe
2
)
6c
5
6d
O
SiMe
3
Me
3
Si
Co
2
(CO)
8
H
(43 %)
CH
2
CH
2
OBzl
CH
2
CH
2
OBzl
OSi-
t
BuMe
2
OSi-
t
BuMe
2
6b
7
O
O
SiMe
3
Me
3
Si
Me
3
Si
Co
2
(CO)
8
H
H
OBzl
H
H
OBzl
+
OBzl
dr 3:1 8a:9a
yield 31 %
Me
2
t
Bu-SiO
Me
2
t
Bu-SiO
OSi-
t
BuMe
2
6c
8a
9a
O
O
SiMe
3
Me
3
Si
Me
3
Si
Co
2
(CO)
8
H
OBzl
H
OBzl
H
+
OBzl
OSi-
t
BuMe
2
H
dr 1:1 8b:9b
yield 33 %
Me
2
t
Bu-SiO
Me
2
t
Bu-SiO
6d
8b
9b
Scheme 4.2
Diastereoselective dicobaltoctacarbonyl-mediated cyclization of the acyclic
enynes
6a-d
described by Mulzer
et al.
H
(OC)
3
Co
Co(CO)
3
SiMe
3
H
R
1
O
5
6
R
2
SiMe
3
R
1
O
H
H
2
1
(OC)
3
Co
Co(CO)
3
R
2
10
11
Scheme 4.3
Transition state models
10
and
11
.
In 1991, Roush
et al.
reported the enantioselective crotylboration of 3-decynal dicobalt
hexacarbonyl
12
. This reaction provides a very convenient route to stereochemically defined
1,6-enyne dicobalt hexacarbonyl complexes
13
and
14
, which undergo a highly diastereos-
elective intramolecular PKR to form bicyclooctenones
15
and
16
(Scheme 4.4).
4