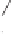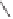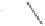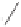Chemistry Reference
In-Depth Information
R
R
0.1 g of Co/charcoal (cobalt 12 wt.%)
Z
O
Z
THF, 130 °C, CO (20 atm)
Z = C(CO
2
Et)
2
, R = H: 91%
Z = C(CO
2
Et)
2
, R = Me: 98%
Z = NTs, R = H: 98%
Z = O, R = Ph: 98%
(1.26 mmol)
O
R
R
The same as above
+
(1.64 mmol)
R = Ph: 98%
R = n-C
5
H
11
: 98%
R = cyclohex-1-enyl: 98%
Scheme 3.27
Chung disclosed colloidal cobalt nanoparticles and the cobalt nanoparticles on charcoal
as other examples of reusable heterogeneous catalysts. The former catalyst was prepared by
stirring Co
2
(CO)
8
, oleic acid, trioctylphosphine and dioctyl ether under the reflux condition,
and was precipitated by adding ethanol to the cooled reaction mixture.
27
Refluxing the
prepared colloidal cobalt nanoparticles with charcoal provided the latter catalyst.
28
Intra-
and intermolecular reaction proceeded under a lower CO pressure of 5 atm but at a high
temperature (130
◦
C) by using these heterogeneous catalysts.
As for other choices of heterogeneous catalyst, Leitner used Raney cobalt for the catalytic
reaction (Scheme 3.28).
29
It is commercially available and readily accessible, but requires
a high pressure of carbon monoxide (23 atm) and high reaction temperature (130
◦
C), and
catalytic activity was significantly decreased by twice recycling.
R
R
Raney cobalt (6 mol%)
Z
O
Z
THF, 130 °C, CO (23 atm)
Z = C(CO
2
Et)
2
, R = H: 91%
Z = C(CO
2
Et)
2
, R = Me: 94%
Z = C(CH
2
OH)
2
, R = H: 92%
O
R
Raney cobalt (17 mol%)
R
+
THF, 130 °C, CO (23 atm)
R = Ph: 86%
R = n-C
3
H
7
: 56%
R = (CH
2
)
3
OH: 52%
Scheme 3.28