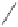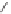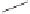Chemistry Reference
In-Depth Information
Groth used a microwave in place of heating.
25
The reaction concluded within 5 min at
100
◦
C in the presence of relatively large amounts of Co
2
(CO)
8
along with a stoichiometric
amount of cyclohexylamine (Scheme 3.25). The intermolecular reaction of several alkynes
gave acceptable yields, but only an example of an intramolecular reaction with a moderate
yield (46%) was listed.
R
1
Co
2
(CO)
8
(20 mol%)
O
CyNH
2
(120 mol%)
R
1
+
Toluene, 100 °C, CO (1 atm)
R
2
Microwave 5 min
R
2
R
1
= Ph, R
2
= H: 81%
R
1
= n-C
6
H
13
, R
2
= H: 72%
R
1
= i-Pr, R
2
= H: 71%
R
1
= R
2
= CO
2
Me: 48%
Scheme 3.25
3.7 Catalytic Reaction Using Heterogeneous Catalysts
Several examples of co-catalyzed Pauson-Khand reactions in heterogeneous conditions are
listed in this section. Chung disclosed the pioneering work by using metallic cobalt on
mesoporous silica (SBA-15) with 9-10 wt.% cobalt content.
26
A high reaction temperature
(130
◦
C) and high pressure of carbon monoxide (20 atm) were required, but an intramolec-
ular reaction of enynes proceeded smoothly to give carbonylative cycloadducts in a high to
excellent yield (Scheme 3.26). Moreover, three-times recycling of the heterogeneous cata-
lyst was ascertained without loss of catalytic activity. This catalyst was less effective for the
intermolecular reaction of norbornene derivatives with phenylacetylene (11-36% yield).
R
R
0.1 g of Co/SBA-15 (cobalt 9-10 wt.%)
CH
2
Cl
2
, 130 °C, CO (20 atm)
Z
Z
O
Z = C(CO
2
Et)
2
, R = H: 98%
Z = C(CO
2
Et)
2
, R = Me: 92%
Z = NTs, R = H: 88%
Z = O, R = Ph: 88%
(1.26 mmol)
Scheme 3.26
Chung further realized a more convenient and less expensive heterogeneous system using
cobalt on charcoal.
27
The reaction conditions were still harsh, but nine-times recycling
without loss of catalytic activity could be possible, moreover, this catalyst could also
operate in the intermolecular reaction of norbornadiene with terminal alkynes (Scheme
3.27).