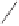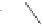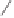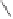Chemistry Reference
In-Depth Information
R
R
R
R
(OC)
3
Co
Co(CO)
2
(OC)
3
Co
Co(CO)
3
(OC)
3
Co
Co(CO)
2
Co
2
(CO)
6
-CO
HO
H
HO
HO
O
R
O
CO
R
(OC)
3
Co
Co(CO)
2
R
O
-[Co
2
(CO)
6
]
Co
2
(CO)
6
H
O
Scheme 3.9
Soon after this publication, Jeong used triphenylphosphite as an additive for Co
2
(CO)
8
and achieved the first practical and catalytic Pauson-Khand reaction.
9
Various 1,6-enynes
were transformed into the corresponding bicyclic cyclopentenones at 120
◦
C under a slightly
pressurized condition of carbon monoxide (Scheme 3.10).
Co
2
(CO)
8
(3-5 mol%)
P(OPh)
3
(10-20 mol%)
Z
R
Z
O
DME, 120 °C
CO (3 atm)
R
Z = C(CO
2
Et)
2
, R = H: 82%
Z = CMe
2
, R = H: 77%
Z = C(CO
2
Et)
2
, R = CH
2
OBn: 82%
Z = NTs, R= H: 94%
Scheme 3.10
Later, triphenylphosphine was found to be an effective additive, but the ratio against
Co
2
(CO)
8
was very important (Scheme 3.11).
10, 11
The heptacarbonyl (triarylphos-
phine)dicobalt complex, where only one of CO ligands was displaced with triphenylphos-
phine, was an effective catalyst, but the hexacarbonyl bis(triarylphosphine)dicobalt complex
had almost no catalytic activity, as previously mentioned above.
Co
2
(PAr
3
)(CO)
7
(5 mol%)
TsN
O
TsN
THF, 50 °C
CO (1.05 atm)
Ar = Ph: 84%
p-MeOC
6
H
4
: 91%
Scheme 3.11
Hashimoto and Saigo reported tributyl phosphine sulfide as an efficient ligand for the
catalytic reaction (Scheme 3.12). The effect of sulfur is unclear, but triphenylphosphine
oxide was less effective. The carbonylative reaction of various enynes proceeded at lower
temperature (70
◦
C) under ambient pressure of carbon monoxide to give bicyclic enones in
a good to high yield.
12
An intermolecular reaction also proceeded under the same reaction
conditions to give a tricyclic enone in high yield.