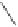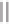Chemistry Reference
In-Depth Information
O
Co
2
(CO)
8
(9 mol%)
Acetylene
then acetylene:CO (1:1)
+
2,2,4-trimethylpentane
60-70 °C
74%
Scheme 3.6
Another example required more harsh reaction conditions (Scheme 3.7). In the presence
of a catalytic amount of Co
2
(CO)
8
, alkyl acetylene reacted under the highly pressurized
condition of ethylene and carbon monoxide at a high temperature.
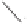
5
O
n-C
5
H
11
Co
2
(CO)
8
(0.22 mol%)
n-C
5
H
11
+
Ethylene
150 °C, CO (ca. 100 atm)
(ca. 40 atm)
49%
Scheme 3.7
The substrate scope of the above two examples was narrow, and the reactions were
practically premature, but they showed the possibility of catalytic reaction.
3.4 Catalytic Reactions by Aid of Additives
The “appropriate” stabilization of Co
2
(CO)
6
, which possibly forms in the reaction pathway,
was considered important for the achievement of a catalytic reaction. Displacement of
the CO ligand with triarylphosphine generally stabilizes the cobalt carbonyl complex, but
Co
2
(CO)
6
(PPh
3
)
2
is too stable and has little catalytic activity.
The avenue for the catalytic Pauson-Khand reaction was brought about by another reac-
tion using Co
2
(CO)
8
: Iwasawa reported the Co
2
(CO)
8
-mediated transformation of alkynyl-
cyclopropanols into cyclopentenones.
6
He further examined the catalytic version of this
rearrangement and found that bulky phosphite was effective as an additive (Scheme 3.8).
7
R
O
Co
2
(CO)
8
(5 mol%)
P[O-o-( i-Pr)C
6
H
4
]
3
(10 mol%)
OH
R
DME, reflux
Scheme 3.8
He speculated on the reaction mechanism as follows: the carbon-carbon bond cleavage
of the cyclopropane ring is an initial step, which would be induced by the electron-donating
effect of the hydroxy group (Scheme 3.9).
8
The mechanism of the second half resembles
that of the Pauson-Khand reaction (Scheme 3.5).