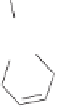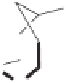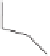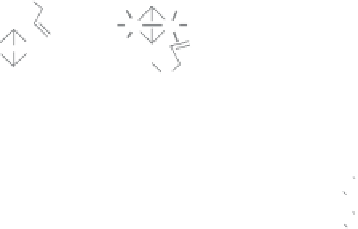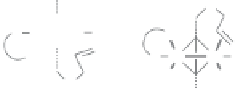Chemistry Reference
In-Depth Information
O
TMS
1. Co
2
(CO)
8
2. Norbornadiene,
NMO·H
2
O
CH
2
Cl
2
, 0 ºC to rt
18 h
O
N
O
2
S
O
H
N
O
2
S
H
Me
3
Si
dr > 800:1
SO
2
O
2
S
N
N
O
N
N
O
O
O
S
S
O
O
OC
O
O
CO
OC
CO
OC
OC
Co
Co
CO
OC
Co
Co
CO
CO
OC
OC
Co
Co
CO
CO
OC
OC
Co
Co
CO
CO
CO
OC
H
H
H
H
B
1eq
(6.7)
B
2eq
(0.0)
C
1eq
(0.0)
C
2eq
(8.9)
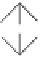
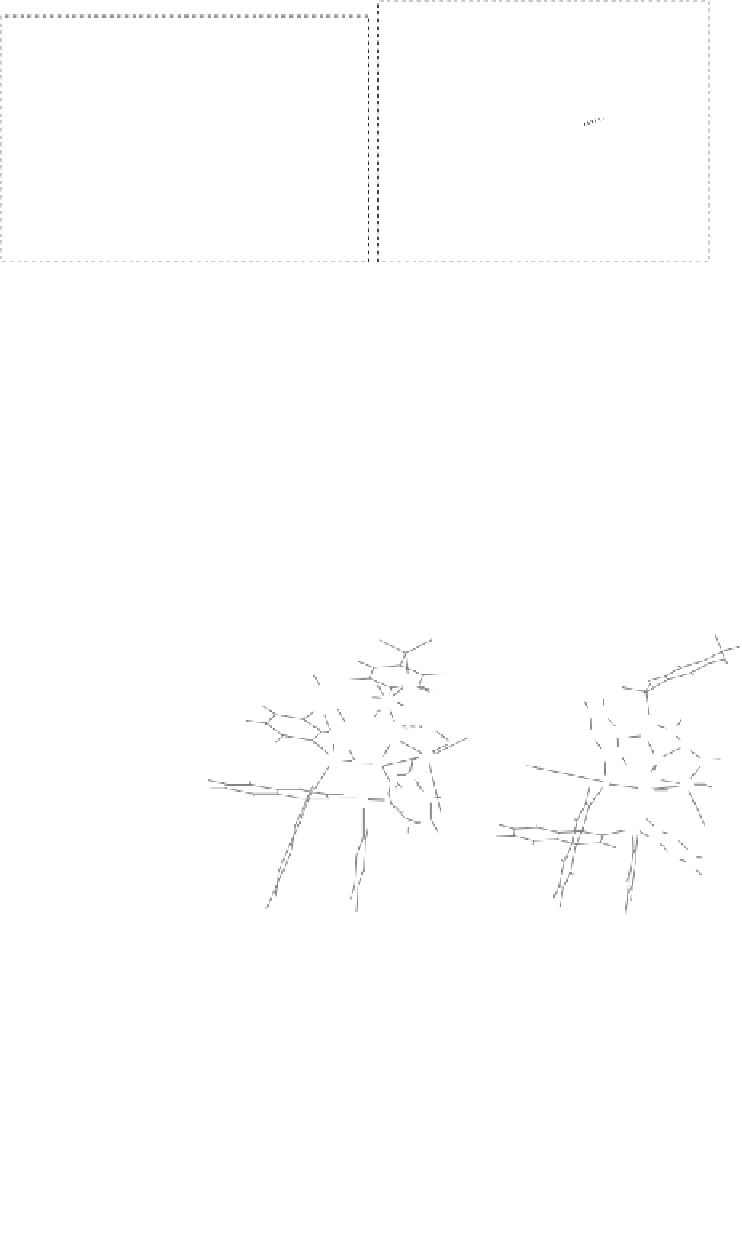
Figure 2.11
Highly diastereoselective PK reaction of (
1R
,
5S
,
7S
)-
N
-(3-trimethylsilylpropiolyl)-
bornane-2,10-sultam and norbornadiene (top) and explanation of the selectivity by the
chelated intermediates
B
(bottom, left) and the olefin complexes
C
(bottom, right). Rela-
tive energies in kcal
mol
−
1
·
are shown between brackets, determined by MP3(tm) (structures
B
) or DFT (structures
C
).
derivatives
31
(Figure 2.11), analysis of the reaction intermediates at the semi-empirical/DFT
level allowed interpretation of the stereoselectivity based on the preference of the sulfur-
containing chelating group to displace one particular carbonyl ligand, thus directing the
olefin coordination to that position.
Very recently, studies on the enantioselective catalytic PKR at DFT level have been pub-
lished. On the one hand, a QM/MM ONIOM approach has been employed for studying the
Co
H
Ts
N
co
P
co
oc
P
Co
Co
oc
co
P
Co
Co
*
co
P
N
*
H
Ts
P
Manifold A
Manifold B
Co
H
Ts
N
oc
co
co
P
Co
Co
P
P
co
co
P
Co
Co
P
oc
Ts
H
TS1
TS1'
Manifold C
Manifold D
Figure 2.12
The four possible manifolds studied in the intramolecular enantioselective PKR
with BINAP as a ligand (left) and the two lowest transition states, both from Manifold A, giving
place to the
R
(
TS1
, 0 kcal
mol
−
1
)and
S
(
TS1'
, 9.0 kcal
mol
−
1
) products.
·
·