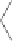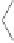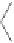Chemistry Reference
In-Depth Information
Intramolecular PK reactions on chiral dienynes giving place to bicyclo-[3.3.0]-octenones
have been rationalized on the basis of the relative energies of the intermediate metallacy-
cles (
D
) obtained upon olefin insertion, calculated at a MM level. This method, although
extremely simple, allows to correctly reproduce some of the experimental results obtained
with this kind of substrates
26
(Table 2.2).
Also, computational studies were used to explain the selectivity obtained in the
intermolecular reaction with alkynes bearing camphor-derived chelating chiral auxiliaries.
Both for substituted thioisoborneol
19c
(Figure 2.10) and for Oppolzer's camphorsultam
HS
HS
HS
O
O
O
O
O
OC
S
OC
CO
OC
CO
OC
CO
OC
OC
OC
Co
Co
CO
OC
OC
Co
Co
S
OC
OC
Co
Co
CO
OC
OC
Co
Co
OC
OC
Co
Co
CO
CO
CO
CO
CO
H
H
H
H
H
B
1eq
(5.9)
B
1ax
(0.0)
C
1eq
(3.9)
C
1ax
(2.2)
C
1eq
(0.0)
HS
HS
HS
O
O
O
O
O
OC
S
CO
OC
CO
CO
OC
CO
CO
OC
OC
Co
Co
CO
S
OC
Co
Co
CO
OC
Co
Co
CO
CO
Co
Co
CO
CO
OC
OC
Co
Co
CO
CO
CO
CO
OC
H
H
H
H
H
B
2eq
(1.0)
B
2ax
(3.6)
C
2eq
(1.8)
C
2ax
(4.7)
C
2eq
(2.3)
O
H
(OC)
2
Co
Co(CO)
3
(OC)
2
Co
Co(CO)
3
H
H
D
1anti
B
1eq
C
1ax
RO
H
H
O
O
H
H
H
Minor
HS
HS
D
1anti
(1.0)
D
1syn
(14.5)
(OC)
3
Co
Co(CO)
2
(OC)
3
Co
Co(CO)
2
H
H
O
H
H
H
O
O
D
2anti
C
2eq
B
2ax
RO
H
H
SH
SH
H
D
2anti
(0.0)
D
2syn
(12.3)
Major
Figure 2.10
Study of the stereoselective PK reaction of
O
-alkynyl-(2
R
)-10-mercaptoi-
soborneol with norbornadiene. Top, left: Dicobaltpentacarbonyl alkyne complex
B
, with the
thiol group coordinating one of the Co atoms. Top, right: Alkene complex
C
, after substitution
of the thiol by norbornadiene. Bottom, left: Cyclometalated complexes
D
, after alkene insertion
into the acetylenic C-Co bond. Bottom, right:
Genealogy
of the two observed products, ac-
counting for the observed selectivity. Numbers in brackets are relative energies in kcal
mol
−
1
,
·
obtained from single point calculations at DFT level on PM3(tm)-optimized structures.