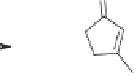Chemistry Reference
In-Depth Information
O
n-C
4
H
9
OHC
n-C
4
H
9
10 mol% [Rh(binap)]BF
4
(CH
2
Cl)
2
80 °C
Me
dihydrojasmone (
135
)
Me
134
83% yield
Scheme 10.39
Tandem hydroacylation/bond migration reaction.
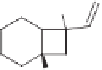
A different approach to the synthesis of cyclopentenones exploits the ring expansion
of vinyl cyclobutanols. Vinyl cyclobutanols, in the presence of palladium(II) catalysts and
2 equivalents of benzoquinone render the desired cyclopentenones. The mechanism is
illustrated in Scheme 10.40, with
137
as the key intermediate. Through this methodology
5,5-, 5,6-, and 5,7-ring systems are readily accessible.
78
HO
PdXL
2
HO
PdX
2
L
2
O
Me
Me
138
137
136
Scheme 10.40
Vinyl cyclobutanols ring expansion of vinyl cyclobutanols catalyzed by Pd.
A similar approach involves the ring expansion of three-membered spiropentane rings that
takes place under an atmosphere of carbon monoxide, and is catalyzed by Rh(I) complexes.
The reaction generates the cyclopentenones with good yields and regioselectivities.
79
O
[RhCl(cod)]
2
5 mol%
DPPP, 10 mol%
p-xylene, CO, 130 °C
56% yield
140
139
Scheme 10.41
Spiropentanes ring expansion catalyzed by Pd.
The formation of cyclopentenone
146
from spiropentane
141
can be explained by the
pathway depicted in Scheme 10.41. Initially, Rh(I) oxidatively adds to the C4-C5 bond
of the corresponding spiropentane to form spirocyclic rhodiacyclobutane
142
. Insertion of
carbonmonoxide generates rhodacyclopentanone
143
. Selective migration of the methylene
carbon (C2) onto rhodium through a
-carbon elimination then converts the spirocyclic
skeleton into the six-membered rhodiacycle
144
. Finally, reductive elimination furnishes
3-methylene cyclopentanone
145
, which isomerizes to the cyclopentenone
146
under the
reaction conditions (Scheme 10.42).
β