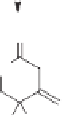Chemistry Reference
In-Depth Information
2
Rh
4
2
CO, Rh(I)
oxidative
addition
3
Rh
CO
R
Me
R
Me
1
R
Me
5
CO insertion
O
143
142
141
-carbon
elimination
β
O
O
O
Rh
R
R
reductive
elimination
isomerization
Me
Me
Me
R Me
145
146
144
Scheme 10.42
Proposed mechanism for the Rh-catalyzed carbonylation of spiropentanes.
The Nazarov reaction employing organometallic reagents is another interesting approach
to the synthesis of cyclopentenones.
11
The Nazarov reaction is formally a cyclization of
divinyl ketones under the influence of very strong acids. In the organometallic version of
this reaction, a metallic Lewis acid such as Cu and Pd, is used instead of a strong Brønsted
acid (Scheme 10.43).
AL
AL
O
O
O
O
R
1
R
1
R
2
R
1
R
2
R
1
R
2
AL
R
2
R
3
R
3
R
4
R
3
R
4
R
3
R
4
150
R
4
149
148
147
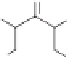
Scheme 10.43
Nazarov reaction.
Although the Nazarov reaction is generally promoted by one or more equivalents of a
Lewis acid (SnCl
4
,TiCl
4
, FeCl
3
or AlCl
3
), the Nazarov reaction catalyzed by transition
metal salts has recently received significant attention. For example, the first palladium-
catalyzed Nazarov cyclization of divinyl ketones was developed by Tius in 2003.
80
The
reaction takes place under mild conditions and leads, in the case of
α
-alcoxy dienones, to
2-hydroxycyclopentenones in good yields (Scheme 10.44).
O
OH
R
1
R
1
OR
2
PdCl
2
(MeCN)
2
O
R
3
R
5
R
3
R
4
R
5
R
6
R
4
R
6
151
152
152a
R
1
=Me R
2
=Et R
3
=Ph R
4
=R
5
=R
6
=H 91% yield
152b
R
1
=R
3
=Me R
2
=Et R
4
=R
5
=R
6
=H 70% yield
152c
R
1
=R
5
=Me R
2
=Et R
5
=Ph R
4
=R
6
=H 92% yield
Scheme 10.44
Nazarov reaction catalyzed by Pd(II).