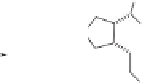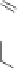Chemistry Reference
In-Depth Information
The desired diastereomer was obtained in a ratio of 88:12. The purified
trans
-
48
was ob-
tained in 57% yield by chromatography. Subsequent reduction of alkene
48
via catalytic
hydrogenation provided an epimeric mixture of ketones
49
. Bayer-Villager oxidation of
49
which employed
m
-CPBA gave the lactone
50
in 86% yield. The lactone ring opening was
effected with Ca(BH
4
)
2
35
in ethanol to give the corresponding diol which was reacted with
TBDMSCl to selectively silylate the primary alcohol to give monol
51
in 92% yield. The
methyl ketone
52
was then obtained from
51
under Ley-Griffith oxidation conditions
36
fol-
lowed by transformation of
52
into the penultimate intermediate
53
with Tebbe's reagent.
37
Jones' oxidation of
53
furnished the dicarboxylic acids and this was followed by removal of
the
N
-BOC protecting group under carefully controlled acidic conditions and ion exchange
chromatography to provide (-)-
α
-kainic acid
54
. The overall yield from carbonate
46
was
12% utilizing the Ir-catalyzed allylic amination reaction and a highly diastereoselective
intramolecular Pauson-Khand reaction.
1. [Ir(COD)Cl]
2
(2 mol %)
(S,S,aS)-L2 (4 mol %)*
TBD (8 mol %)
THF (1.0 M), rt, 24 h (65%)
1) Co
2
(CO)
8
(1.1 equiv)
CH
2
Cl
2,
rt, 4 h
2) Me
3
NO 2H
2
O (6.8 equiv)
4
Å
MS, rt, 4 h
65%
BocN
TBDMSO
OCO
2
Me
H
2
N
TBDMSO
Me
Me
46
(1.2 equiv)
47
(99% ee)
2. Boc
2
O,
n
-Bu
4
NHSO
4
CH
2
Cl
2
/1 M NaOH (1:1)
(93%)
* Chiral ligand
Me
Me
Me
H
H
H
2
(5 bar)
Pd(OH)
2
/C (10 wt%)
EtOAc, rt, 20 h (97%)
m-CPBA (2.5 equiv)
Na
2
HPO
4
(25 equiv)
CH
2
Cl
2
, rt, 9 h (86%)
O
BocN
O
O
BocN
BocN
H
TBDMSO
O
H
H
H
TBDMSO
TBDMSO
48
Trans:cis 88:12
50
49
Me
Me
Cp
2
Ti AlMe
2
Cl
(1.3 Equiv)
pyridine (1.1 equiv)
THF, -40 °C
rt
20 h (68%)
TPAP (10 mol %)
NMO (2. 3 equiv)
4
Å
MS
CH
2
Cl
2
, rt, 1.5 h (90%)
1) CaCl
2
(3 equiv)
NaBH
4
(6 equiv)
EtOH, 50 °C, 4 h
2) TBDMSCl (1.1 equiv)
imidazole (2 equiv)
CH
2
Cl
2
, rt, 5.5 h
(92%)
OH
O
BocN
BocN
TBDMSO
TBDMSO
51
OTBDMS
OTBDMS
52
Me
1) Jones' reagent (5 equiv)
acetone, 0 °C, 20 min
2) CH
2
Cl
2
/TFA (8:1)
rt, 3 h
then DOWEX (H+)
76%
Me
CO
2
H
BocN
CO
2
H
H
TBDMSO
OTBDMS
54
53
(-)-
α
-Kainic acid
Scheme 8.9
Enantioselective total synthesis of (-)-
α
-kainic acid.
8.6 The Total Synthesis of Paecilomycine A
Paecilomycine A is a terpenoid-derived natural product isolated from
Isoria japonica
38
,
Danishshefsky et al. utilized the Diels-Alder reaction with a Danishesky-diene to form the