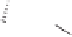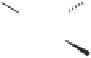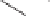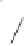Chemistry Reference
In-Depth Information
A-ring and an intramolecular Pauson-Khand reaction to form the bicyclic enone sector.
39
As shown in Scheme 8.10, diene
55
underwent a cycloaddition reaction with dienophile
56
to provide adduct
57
in 86% yield. Subsequent transformations of the endo adduct
57
afforded the eneyne
58
appropriately set-up for the Pauson-Khand cyclization. The alkyne
58
was treated with dicobalt octacarbonyl in the presence of molecular sieves in toluene
at room temperature for 2 hours after which was heated to 100
◦
C for 24 hours to afford
a single stereoisomer
59
in 37% yield. Although the yield was lower than desired, the
reaction was scaleable and the ketone was readily purified.
CHO
Me
OTMS
Me
OTMS
CH
2
Cl
2
+
23 °C
86 %
TMS
55
56
CHO
TMS
57
H
Me
O
Me
O
Co
2
(CO)
8
, 4Å MS
Toluene
37%
1. NaBH
4
/CeCl
3
7H
2
O MeOH
86%
2. n-BuLi, Et
2
O
Et
3
Zn, CH
2
I
2
, benzene
H
TBSO
TMS
TBSO
58
59
53%
O
H
H
H
Me
O
Me
O
Me
O
Me
H
Me
OH
H
5
TBSO
TBSO
O
61
O
OH
OH
60
62
Paecilomycine A
Scheme 8.10
Total synthesis of paecilomycine A.
The key tricyclic adduct
59
was reduced with sodium borohydride to the corresponding
allylic alcohol. The alcohol was converted to cyclopropane
60
to provide the appropriate
functionality for eventual installation of the
β
-methyl group at C(5) based on the earlier
work of Corey et al.
40
which ultimately provided paecilomycine A
62
.
8.7 The Total Synthesis of (
+
)-Achalensolide
Bohlmann [41] isolated (
)-achalensolide
68
from the aerial parts of
Decachaeta thieleana
in 1983. Herz, in the same year, isolated
68
from
Stevia achalensis
, in Copina, Cor-
doba, Argentina.
42
Structurally, (
+
+
)-achalensolide possessed the bicycle[5.3.0]decane ring