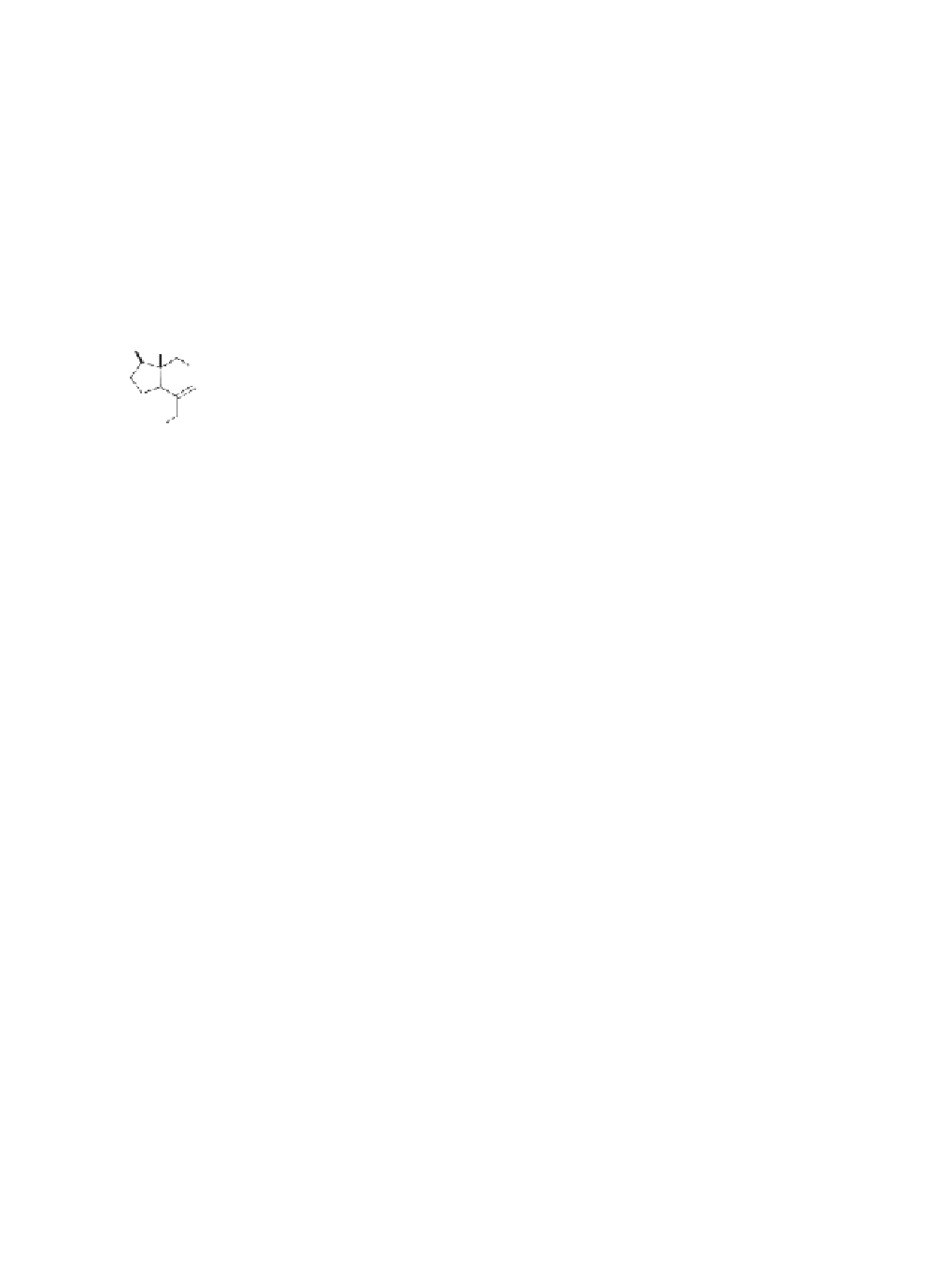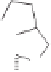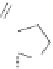Chemistry Reference
In-Depth Information
system. A successful synthesis of this moiety was reported by Schreiber using a Lewis
acid-promoted Nicholas reaction followed by a cobalt-mediated Pauson-Khand reaction
and is shown in Scheme 8.2.
5
(
R
)-Pulegone was chosen as the asymmetric starting material
to synthesize key intermediate
6
. The lithium anion of
7
was reacted through displacement
of a triflate ester derived from alcohol
6
to furnish alkyne
8
in 74% as a 1:1 mixture of
diastereomers at C(10). Treatment of alkyne
8
with dicobalt octacarbonyl provided the
corresponding metallocycle and this was followed by a Lewis-acid mediated cyclization
to provide a mixture of diastereomeric ethers represented by
9
and by-product
10
.The
selectivity of the reaction was 91:9 for
9:10
. Numerous Lewis acids and solvents were
screened and the best conditions were obtained by the use of trimethylsilyl triflate in ether
at
−
78
◦
C to provide good yield and site-selective combination.
Me
Me
H
OH
t
-Bu
Tf
2
O, CH
2
Cl
2
N
t-Bu
H
-10
°
C
Me
H
TMS
1. Co
2
(CO)
8
2. TMSOTf , Et
2
O, -78
°
C
82% (2 steps)
H
-45 to 0
°
C
74%
6
O
Me
Me
OEt
H
TMS
8
n
-BuLi, THF
HMPA, -78
°
C
O
Me
Me
OEt
7
O
O
Co(CO)
3
Me
Me
CHO
H
Me
H
H
Co(CO)
3
H
12
5steps
Me
CH
3
CN, air
ref lux
85%
1. Ph
3
P=CHOMe
2. NaH, toluene
(EtO)
2
P(O)CH
2
CN
Me
H
10
Me
O
Me
O
OR
H
H
H
H
H
H
Me
Me
1
1
9
:R=
(5:1 at C12)
10
:R=Et
NC
OH
NC
OMe
Me
1. Ph
3
,P, imid, I
2
2.
t
-BuLi, Et
2
O
1. HCl, aq. THF
2. NaBH
4
, -78
°
C
91% (2 steps)
H
Me
H
Me
Me
H
H
Me
O
Me
O
H
H
H
H
14
13
CN
Me
Me
H
H
K, 18-crown-6
toluene
82%
Me
Me
H
H
H
Me
Me
O
H
H
H
Me
O
H
H
16
15
(+)-Epoxydictymene
Scheme 8.2
The total synthesis of (
+
)-epoxydictymene.