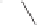Chemistry Reference
In-Depth Information
8.1
Introduction
The total synthesis of target molecules of natural or of computational interest has been a long
standing challenge for the synthetic organic chemist. Starting with simple precursors which
culminate in a successful synthesis has been described as both an art and a science.
1
Not
only are the chemical reactions to form carbon-carbon bonds a challenge, stereochemical
strategies must also be considered when a successful total synthesis is developed. Published
results have benefited both academia and the chemical and pharmaceutical industries for
they have provided key chemical transformations for the benefit of all, as well as new
strategies for molecular construction.
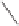
The Pauson-Khand reaction, as described in 1971, became one of the most important
methods for the synthesis of cyclopentenones and bicylic enones.
2
In the stoichiometric
Pauson-Khand reaction, the alkyne is first reacted with dicobalt octacarbonyl to form a
thermally stable alkyne metal complex. When the reaction mixture is heated in the presence
of an alkene and carbon monoxide, this generated cyclopentenones or bicyclic enones in
yields ranging from 40-60%.
3
Basic examples of both inter- and intramolecular Pauson-
Khand reactions are shown in Scheme 8.1. Numerous methods have been developed to
promote the cyclization process catalytically and improve stereoselectivity with various
metals or cyclization processes.
3
O
Co
2
(CO)
6
toluene/heat
H
H
1
2
3
TMS
Co
2
(CO)
8
TMS
O
toluene/heat
CO
4
5
Scheme 8.1
Inter- and intramolecular Pauson-Khand reaction examples.
The scope of this chapter has been restricted to the last 15 years highlighting synthetic
routes in which the Pauson-Khand reaction has been instrumental in the total synthesis
of the chosen target molecule. Noteworthy synthetic examples are showcased in detail,
followed by a section which highlights key Pauson-Khand reactions to divert the reader to
further examples.
8.2
(
+
)-Epoxydictymene
)-epoxydictymene
16,
4
isolated from the brown alga
Dictyota dichomata
,
contains a 5-8-8-5 ring substructure which encases a
trans
-3-oxabicyclo[3.3.0]octane
The diterpene (
+