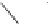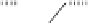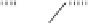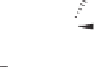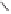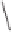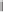Chemistry Reference
In-Depth Information
CO
CO
OC
OC
P
Co
Co
P
S
*
R
R
R
R
Co
Co
S
*
OC
CO
CO
OC
CO
V
IV
Figure 6.1
Possible bonds for olefin insertion in PNSO complexes derived from symmetrically
substituted alkynes: as hemilabile (left) and solid bridging (right).
Although PNSO ligands are usually hemilabile, the high enantiomeric excesses, better
results obtained under N-oxide activation, and long reaction times observed in the reactions
described above led Riera, Verdaguer et al. to propose an alternative mechanism. Another
factor suggesting that the mechanism was not hemilabile was that, in contrast with terminal
alkynes, hemilabile ligands could not direct olefin insertion (i.e. it would not be able to
discriminate between the two Co-C bonds), because the sulfur chirality would be too far
from the reaction center, as shown in
IV
(Figure 6.1). Thus, they suggested that the PNSO
ligand works as a solid bridging ligand, directing olefin coordination to the same cobalt
center to which the sulfinyl moiety is bound, as in
V
(Figure 6.1). Once the olefin has bound
to the cobalt atom, the sulfur chirality directs insertion of the olefin into a single Co-C
bond.
X-ray analysis of the PNSO cobalt complexes
37ia
,
38ib
and
43ia
furnished interesting
clues to the mode of operation of the PNSO ligands.
44
The Co-S bond distance reflects the
bond strength between the sulfur and cobalt atoms and can be used to estimate the hemilabile
character of the ligand. Electron-deficient groups on the sulfur lead to a shorter and stronger
Co-S bond, due to the relatively higher
-acidity of the sulfinyl group (Figure 6.2). Within
the series of PNSO ligand studied, the
t
-Bu-PNSO leads to the weakest sulfur-cobalt bond,
and therefore, is the most hemilabile. Conversely, the CF
3
-PNSO ligand behaves more like a
solid bridged ligand (in other words, less hemilabile). Reactivity studies and CO stretching
frequency measurements corroborate this hypothesis.
The success of PNSO ligands in asymmetric PKRs prompted the Riera and Verdaguer
group to prepare the corresponding analogs with a carbon tether.
45
The sulfinylmethyl
Bn
Bn
i-Bu
CF
3
O
Ph
Ph
OC
N
Ph
Ph
OC
N
Ph
N
P
S
O
P
S
O
P
S
To l
Ph
OC
2.19 Å
2.13 Å
2.17 Å
Co
Co
CO
Co
Co
CO
Co
Co
CO
OC
CO
OC
CO
OC
CO
TMS
H
TMS
H
TMS
H
38ib
37ia
43ia
Figure 6.2
Co-S bond lengths in different PNSO-alkyne cobalt complexes (based on X-ray
analysis).