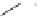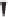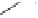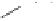Chemistry Reference
In-Depth Information
BH
3
O
BH
3
BH
3
O
O
SP h
Ph
SP h
Ph
SP h
Ph
R
Ph
Ph
Me
45a
45b
44a,
R = H
44b,
R = Ph
44c,
R = Bn
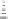
Figure 6.3
Sulfinylmethyl phosphine (PCSO) ligands studied in intermolecular PKRs.
phosphine (PCSO) ligands enabled introduction of an additional chiral center between the
phosphorous and sulfur atoms (Figure 6.3). Synthesis of the ligands
44
and
45
, and studies
on their ligand exchange and PK reactions, revealed that the PCSO ligands are neither as
easy to prepare nor as selective as the PNSO ligands. Nevertheless, this work underscored
the importance of the substitution at the central carbon atom. Compound
44a
did not provide
the bridged P,S complex, whereas ligands
44b
and
44c
did. In ligands
45a
and
45b
, each of
which bears a
tert
-butylsulfoxide group, the chirality of the central carbon atom is crucial
to formation of the bridged species: the desired complex can only be formed if the adjacent
phenyl and
tert
-butyl groups adopt the
trans
conformation due to steric repulsion between
the
tert
-butyl and the phenyl. Thus, whereas
45a
does not provide P,S-bridged complexes,
its corresponding epimer
45b
does.
6.6
Synthetic Applications
Given that only a few practical asymmetric PK methodologies have been developed, enan-
tioselective PKRs of complex molecules are scarce. The PK cycloadduct
3i
,formedfrom
trimethylsilylacetylene and norbornadiene, has proven to be the most synthetically useful
to date. It can be prepared in high optical purity and on a multi-gram scale using either
PuPHOS or PNSO ligands. Using PuPHOS, the diastereoselectivity in the ligand exchange
reaction is 3:1, but gram scale amounts of diastereomerically pure compound can be
obtained through a dynamic resolution process in which crystallization and re-equilibration
steps are alternatively employed (Scheme 6.26);
46
in fact, the major diastereomer can be
obtained in 66% overall yield after two cycles. The crystalline complex gives the PKR with
norbornadiene in 96% yield and 96% ee, which can easily be increased by recrystallization.
Working with the ligand prepared from natural pulegone, the Riera and Verdaguer
group obtained the dextrorotatory enantiomer, whose absolute configuration is shown in
Scheme 6.26.
The PNSO ligands offer three main advantages over the PuPHOS ligands: they are
much easier to prepare, offer higher diastereoselectivity and both enantiomers are equally
available. When cobalt complex
1i
(prepared from trimethylsilylacetylene) was reacted
with ligand
35b
, the bridging complex
37ib
was formed in 67% yield and with a di-
astereoselectivity of 14:1 (Scheme 6.27). Therefore, this process does not require a second