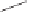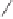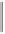Chemistry Reference
In-Depth Information
Tab l e 6 . 4
Asymmetric PKRs of norbornadiene and cobalt tetracarbonyl complexes of (P,S)
bidentate ligands.
O
*
PS
CO
OC
R
Co
Co
OC
CO
Conditions
R
31
3
SM
∗
R
Ligand
de
∗∗
Conditions
Product
Yield
ee
31ia
TMS
PuPHOS
100% toluene/50
o
C
3i
92% 57%
31ia
TMS
PuPHOS
100% CH
2
Cl
2
/NMO/rt
3i
93% 97%
31id
TMS
MeCamPHOS
85% C
6
D
6
/65
◦
C
3i
68% 56%
31id
TMS
MeCamPHOS
85% CH
2
Cl
2
/NMO/rt
3i
72% 79%
31ca
CMe
2
OH PuPHOS
100% CH
2
Cl
2
/NMO/rt
3c
98% 70%
31cd
CMe
2
OH MeCamPHOS
86% CH
2
Cl
2
/NMO/rt
3c
90% 50%
31ka
CONEt
2
PuPHOS
100% toluene/65
o
C
3k
33% 90%
31ka
CONEt
2
PuPHOS
100% CH
2
Cl
2
/NMO/rt
3k
81% 73%
31kc
CONEt
2
CamPHOS
100% toluene/DMSO/70
o
C
3k
68% 92%
31kc
CONEt
2
CamPHOS
100% CH
2
Cl
2
/NMO/rt
3k
81% 80%
∗
Starting material;
∗∗
Diastereomeric excess of the starting complex
The P,S ligand systemwas further evolved with the stereoselective oxidation of the sulfur
in CamPHOS to afford the novel sulfoxide-phosphine ligand
32
40
(Scheme 6.23). During
tests on its coordination capacity, ligand
32
was found to react with the alkyne dicobalt
complex
13
(already used for coordination with chiral sulfides; see Scheme 6.11). The
X-ray structure of the resulting complex (
33
) showed a bridged P,S coordination to cobalt
by the lone pair at the sulfur and the phosphorous atom. Coordination of the sulfoxide to
cobalt set the stage for yet another generation of chiral P,S ligands, in which the chiral
information resides at the sulfur atom.
CO CO
OC
CO
Co
Co
OC
CO
O
Ph
t-BuO
2
S
SO
2
t-Bu
Ph
P
S
13
BH
3
m-CPBA
BH
3
O
O
O
OC
OC
S
P
Ph
Ph
S
P
Ph
Ph
CO
CO
DCM, rt
78%
DABCO, toluene, 70 °C
Co
Co
O
H
H
t
-BuO
2
S
SO
2
But
CamPHOS·BH
3
85%
32
33
Scheme 6.23
Oxidation of CamPHOS, and subsequent coordination of the product to hex-
acarbonyl dicobalt bis(tert-butylsulfonyl)acetylene (
13
).