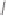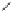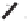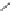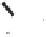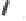Chemistry Reference
In-Depth Information
Tab l e 6 . 3
Enhancement of the diastereoselectivity of hemilabile ligands through hydrogen
bonding interactions.
H
O
S
OC
OC
P
O
P
S
CO
CO
Co Co
H
OC
CO
Co
Co
OC
CO
O
H
*
P
S
O
H
R
2
N
OC
CO
CO
R
2
N
OC
BH
3
Co
Co
31
OC
CO
DABCO
Toluene, 65 °C
O
H
NR
2
O
S
OC
OC
P
O
P
S
CO
1k-m
CO
H
Co
Co
OC
Co
Co
CO
OC
CO
O
H
O
H
NR
2
NR
2
31'
SM
∗
R
Ligand
Product
dr (31/31')
1k
Et
PuPHOS
31ka
32:1
1k
Et
CamPHOS
31kc
19:1
1k
Et
TolCamPHOS
31kg
200:1
1k
Et
CyCamPHOS
31ke
3.1:1
1k
Et
t-BuCamPHOS
31kf
3.1:1
1l
i
-Pr
PuPHOS
31la
19:1
1l
i
-Pr
CamPHOS
31lc
200:1
1m
−
(CH
2
)
5
-
PuPHOS
31ma
7.3:1
1m
−
(CH
2
)
5
-
CamPHOS
31mc
200:1
∗
starting material
intermolecular PK adduct
3l
was obtained in high yield (Scheme 6.22). Although the
induction level attained (23%) was still far from what would be considered synthetically
useful, this method offers the best overall results (i.e. yield plus ee) achieved to date.
O
O
H
O
O
31Ic
5% mol
S
PPh
2
+
OC
CO
CO 2 bar
Toluene, 70 °C
(i-Pr)
2
N
(i-Pr)
2
N
Co
Co
OC
CO
H
O
3I
91% yield
23% ee
31Ic
(i-Pr)
2
N
Scheme 6.22
Catalytic asymmetric PKR of diisopropylpropynamide and norbornadiene.