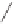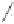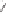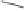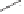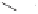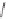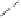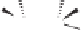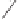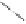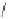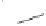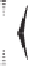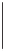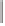Chemistry Reference
In-Depth Information
R''
O
OC
CO
OC
CO
O
O
H
R'
P
OC
CO
OC
CO
L*
Co
Co
Co
Co
*L
Ph
OC
CO
L*
Toluene
Toluene
80 °C
Ph
Ph
R''
H
80 °C
L*
1h
30-75% yield
2h
(13-38% ee)
R' = OPh, OCH(CF
3
)
2
,
NMe
2
, NEt
2
, N(i-Pr)
2
R'' = H, Me, Ph
Scheme 6.17
Asymmetric PKR based on double substitution of a dicobalt hexacarbonyl
complex with a monodentate chiral phosphoramidite ligand.
library of chiral phosphines used for the intermolecular PKR against the archetypical
phenylacetylene-norbornene diad, obtaining less than 10% ee with each phosphine
19a
(Scheme 6.18). In a more recent report, reaction of high nuclearity cobalt clusters with
various chiral phosphines gave little or no induction.
32
H
O
0.2 mol Co
2
(CO)
8
0.2 mol phosphine
H
CO
(OC)
3
Co
Co
Ph
P
Ph
CO, DME
Δ
Co
CO
OC
H
P
CO
2h
(< 10% ee)
Scheme 6.18
Catalytic intermolecular PKR with bis(phosphines) as ligands: poor enantiose-
lectivities are observed, even when other chiral cobalt clusters (left) are employed.
6.5.4
C
2
-Symmetric Bridging Bis-phosphines
The advantage of using
C
2
-symmetric dicobalt-alkyne intermediates was first exploited by
Meijere in the context of diastereoselective reactions using
C
2
-chiral auxiliaries.
33
Prior
to their studies on monodentate phosphoramidites, Greene, Gimbert et al. described a
ligand-based route to such precursors that avoids formation of diastereomeric complexes.
They reasoned that successful implementation of
C
2
-symmetric ligands would require a
single atom spacer between the Lewis basic sites in order to enforce a bridged coordination
with the cobalt cluster (as bridging would result in a five-membered cobaltacycle, whereas
chelation would lead to a four-membered ring). To overcome the (reported) lack of reac-
tivity of bis(phosphine) bridged dicobalt clusters, they focused on less electron-donating
aminophosphines and phosphoramidites. The resulting complexes showed reasonable re-
activities, providing PK adducts in good yields. Preliminary results with a ligand derived
from
-methylbenzylamine displayed some asymmetric induction (Scheme 6.19). How-
ever, neither tuning the stereoelectronic properties of the ligand, nor incorporating chirality
in the phosphine moiety (in the form of a BINOL-derived phosphoramidite), offered any
significant improvement in the results.
31