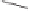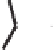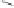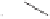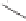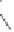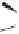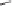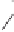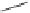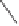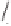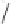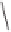Chemistry Reference
In-Depth Information
*
P
P
OC
OC
O
R
PPh
2
PPh
2
Co
Co
*
OC
CO
=
P
P
2
R
R = Ph,
t
-Bu
CO
CO
OC
P
Co
Co
OC
OC
*
CO
P
OC
C
C
P
Co
Co
O
O
OC
TsN
*
TsN
O
P
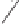
20a
19a
TsN
55 %, 88 % ee
Scheme 6.14
Bridged
vs.
chelated
bis(phosphine) cobalt-alkyne complexes in the PKR.
challenging alkene substrate, 2,5-dihydrofuran, was employed in a subsequent study, offer-
ing moderate success.
28
Kerr and co-workers later combined this approach with
N
-oxide
activation,
29
observing higher yields and good enantiomeric excess for several cyclopen-
tenones.
H
O
PPh
2
O
OC
CO
OC
CO
OC
CO
OC
CO
CO
OC
CO
OC
GLYPHOS (L*)
Co
Co
Co
Co
Co
Co
+
OC
* L
CO
OC
L*
CO
Ph
Ph
Ph
1h
21
dr 6:4
21'
O
T
= 45 °C, 100% ee
= 90 °C, 90% ee
Ph
OC
CO
T
OC
CO
Toluene
2h
Co
Co
* L
CO
O
Ph
O
21
O
Ph
59% ee
59 °C, SiO
2
22
Scheme 6.15
Ligand exchange and PKRs with GLYPHOS.