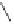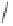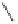Chemistry Reference
In-Depth Information
yield a pair of enantiomers, indicating that the cobalt vertices are prochiral (enantiotopic)
(Scheme 6.3). However, if the ligand is chiral (
L
∗
), then two diastereomeric complexes
arise. Using symmetric alkynes initially simplifies the stereochemical challenge: coordina-
tion of an achiral ligand yields a single achiral complex whereas the use of a chiral ligand
provides a single chiral complex (Scheme 6.3). In this case, once the olefin has coordinated
to the unsubstituted cobalt, diastereoselective insertion of the olefin into one of the two
(almost-equally hindered) Co-C bonds becomes much more difficult, as we will discuss
later on.
L
L
(OC)
3
Co
Co(CO)
2
(OC)
2
Co
Co(CO)
3
R
R'
R
R'
L
R
R'
R = R'
≠
enantiomers
single achiral compound
- CO
(OC)
3
Co
Co(CO)
3
L*
R
R'
- CO
L*
L*
(OC)
3
Co
Co(CO)
2
(OC)
2
Co
Co(CO)
3
R
R'
R
R'
R
≠
R'
diastereomers
R = R'
single chiral compound
Scheme 6.3
Formation of stereoisomeric dicobalt-alkyne complexes with an arbitrary ligand L.
In summary, diastereoselective coordination of the olefin to one of the two enantiotopic
cobalt atoms is one of various requirements for any enantioselective PKR. In the following
sections, we cover different versions of the asymmetric PKR. Rather than following a strict
chronological order, we explore them according to the approach used for desymmetrization
of the alkyne-dicobalt cluster.
6.3
Intrinsically Chiral Dicobalt Clusters
6.3.1 Resolution of Monophosphine Complexes
The groups of Chung
7
and Kerr
8
have described preparation of intrinsically chiral di-
cobalt complexes bearing
achiral
phosphines using a chiral auxiliary approach. Reaction
of the hexacarbonyl dicobalt complex of a chiral acetylene (menthyl propargyl ether [
1a
]
or phenylprop-2-yn-1-ol [
1b
]) with simple phosphines or phosphites affords a mixture of