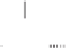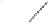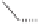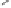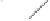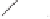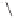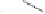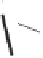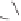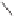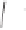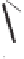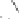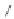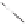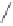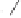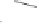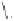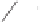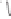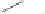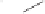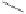Chemistry Reference
In-Depth Information
form two cobaltacycles (
anti-IIIA
and
syn-IIIA
;or
anti-IIIB
and
syn-IIIB
, respectively).
Interestingly,
syn IIIA
and
anti-IIIB
would provide one enantiomer, whereas
anti-IIIA
and
syn-IIIB
would provide the opposite one. However, molecular orbital calculations
have shown that
anti
cobaltacycles are much more stable than
syn
cobaltacycles
6
(for
a detailed mechanistic discussion, see Chapter 2). In summary, enantioselective PKRs
have three main requirements: stereoselective coordination of the olefin to one of the two
cobalt atoms; regioselective insertion of the olefin into one of the two Co-C bonds; and
diastereoselective insertion through an
anti
or
syn
mode.
Co
H
R
Co
H
syn-IIIB
O
H
Co
R
H
Co
H
R
Co
R
H
Co
IIB
H
CO
CO
OC
anti-IIIB
Co
R
H
Co
OC
CO
H
OC
Co
I
Co
R
O
H
R
H
Co
H
Co
R
anti-IIIA
H
IIA
H
Co
R
Co
H
syn-IIIA
Scheme 6.2
Stereochemical mechanistic pathways leading to each enantiomer of
the
cobaltacycle
III
. Carbonyl ligands on intermediates have been omitted for clarity.
Since coordination of a Lewis base into the dicobalt cluster nearly always drives olefin
coordination to (and subsequent insertion at) the distal cobalt center, diastereoselective
coordination with a ligand is extremely important in a ligand-based strategy, especially
for catalytic applications. Although turnstile rotation can enable ligand exchange between
equatorial and axial positions within a cobalt vertex, this is irrelevant to the stereochemistry
of the complexes. In contrast, exchange between cobalt sites may occur at elevated tempera-
tures, leading to isomerization processes that are often deleterious for efficient asymmetric
induction into the final PK adduct. Interestingly, when a CO is replaced with a differ-
ent ligand (
L
), dicobalt complexes of terminal or asymmetrically disubstituted acetylenes