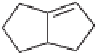
Chemistry Reference
In-Depth Information
leading to the recovery of the chiral auxiliary too. Since the absolute configuration of
3
at C5
was known to be (
R
), that at C5 of themajor diastereomer of
1b
could also be assigned as (
R
).
This stereochemical outcome could be rationalized in the framework of the currently ac-
ceptedmechanism for the PKR (see Scheme 5.4 above). Molecular modeling studies showed
that the most stable conformation of dicobalt hexacarbonyl complexes of alkoxyethynes
derived from
trans
-2-phenycyclohexanol is that in which the bulky [
-Co
2
(CO)
6
] group is
disposed as far as possible from the cyclohexane ring. The analogous conformation in the
case of the enynes leading to
1b
and
1c
(Scheme 5.11) places the pro-(
S
) cobalt atom so
as to be more accessible to the alkene than the diastereotopic pro-(
R
) one due to shielding
by the phenyl group. Assuming that the metallocycle formation is the step that controls the
diastereoselectivity of the process, the major diastereomer of
1b
would then arise from a
cis
-cobaltacycle, that is the product of coordination of the pro-(
S
) cobalt with the
Si
face
of the olefin. Coordination of the pro-(
S
) cobalt with the
Re
-face of the olefin would lead
to a less stable
trans
-cobaltacycle. In the case of
1c
, the energy difference between the
cis
-
and the
trans
-cobaltacycles would be diminished due to the steric interaction between the
methyl and the exocyclic Co(CO)
3
moiety, leading to a reduced diastereoselectivity in the
cyclization. It is interesting to note that molecular mechanics calculations on the proposed
cobaltacycle intermediates give support to this interpretation.
17
O
O
Ph
Ph
O
Co(CO)
3
Co(CO)
3
CO
CO
O
Co
Co
(CO)
3
R
R
R
(R = Me)
(Major)
O
O
Ph
Ph
O
Co(CO)
3
R
Co(CO)
3
CO
CO
O
Co
Co
(CO)
3
R
R
(Minor)
Scheme 5.11
Subsequently, it was found that the intramolecular PKR of ynol ethers of camphor-
derived neopentyloxy alcohols
4a
and
4b
took place in milder conditions, leading to
higher yields and diastereoselectivities.
18
As depicted in Scheme 5.12, both alcohols were
converted to the propargyl alcohols
5a
and
5b
, respectively, by trapping of the
in situ
generated alkoxyethynyl lithium derivatives
14
with para-formaldehyde. Allylation of these
compounds provided the corresponding enynes
6a
and
6b
in good overall yields.
The cobalt-mediated bicyclization of these alkoxyenynes readily took place (Table 5.2).
When
6a
was subjected to the thermal PKR conditions at 50
◦
C,
7a
was obtained as a