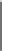Chemistry Reference
In-Depth Information
Tab l e 5 . 1
R*O
Co
2
(CO)
8
R*O
O
R
Xylene or isooctane, reflux,
4-16 h
R
1a
-
c
Product
R*O-
R
Yield (%)
Dr
Me
O
1a
30
1.1:1
H
Me
Me
Ph
1b
H
38
3.2:1
O
Ph
1c
Me
28
1.2:1
O
1b
, whose enyne precursor was obtained in 90% yield from (1
S
,2
R
)-2-phenylcyclohexanol
and 5-iodopentene by the procedure depicted in Scheme 5.9.
14
The extent of the diastereos-
election, however, was observed to be a function of the olefin substitution, and the methyl-
substituted bicyclooctenone adduct
1c
was produced with very low diastereoselectivity.
The configuration of the newly created stereogenic center in the major diastereomer of
1b
could be established as shown in Scheme 5.10. The major isomer of
1b
, obtained in di-
astereomerically pure form after chromatographic purification, was exposed to Yamamoto's
methylcopper-boron trifluoride reagent to give the conjugate addition product
2b
(as a mix-
ture of epimers at C2). Reductive cleavage of the auxiliary in
2b
with samarium diiodide
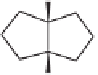
then cleanly produced the known (
+
)-
cis
-1-methylbicyclo[3.3.0]octan-3-one
3
(
95% ee),
Ph
Ph
O
O
Me
Me
SmI
2
, MeOH,
MeCu·BF
3
(3 equiv)
O
O
O
5
5
5
Et
2
O, -78 °C to rt, 3 h
THF, -78 °C
H
H
H
1b
(major)
2b
3
Scheme 5.10