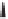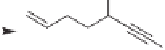Chemistry Reference
In-Depth Information
148
with high enantiomeric purity.
39
In contrast to other studies concerning the PKR of
enantiomerically enriched propargylic alcohols, which featured additional chiral centers in
the PK substrates in order to isolate the enantiomers and contribute to the stereocontrol
of the reaction, two types of chiral enynes
147, 148
, were synthesized and observed to
undergo highly diastereoselective intramolecular PK cycloaddition with retention of their
enantiomeric purity. Corresponding optically active 5,5- and 5,6-fused bicyclic products
149
,
150
were obtained in good to high yields (up to 94%) with high diastereoselectivity (dr
up to 99:1). In the major product, the propargylic substituent and the bridgehead hydrogen
are located cis to each other on the fused bicyclic systems (Scheme 4.37).
XO
R
OX
O
∗
Co
2
(CO)
8
(
S
)-BINOL or (
S
)-H
8
BINOL
O
R
+
n
n
H
NMO
n
ZnEt
2
,Ti(O
i
Pr)
4
,additive
R
H
147
(n = 1)
148
(n = 2)
149
(n = 1)
150
(n = 2)
R=Ph,-CH
2
Ph, -CH
2
CH
2
Ph,
-CH
2
CH
2
CH
2
CH
3
,CO
2
Me, TMS
X=H,Ac,Me
dr up to 99:1
ee up to 95 %
Scheme 4.37
Highly enantio- and diastereoselective Pauson-Khand reactions of enantiomer-
ically enriched propargylic alcohols.
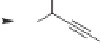
Similar results were obtained with allylic ethers of chiral propargylic alcohols
151
:they
also underwent a highly diastereoselective PKR and retained their high enantiomeric purity
(Scheme 4.38). This study demonstrated that the sizes of the substituents at the propargylic
position and on the alkyne are important for diastereoselectivity, as more bulky substituents
resulted in higher diastereoselectivity.
OH
O
∗
O
(
S
)-BINOL
Br
Co
2
(CO)
8
NMO
∗
∗
O
∗
O
R
+
R'
R'
H
R'
ZnEt
2
,Ti(O
i
Pr)
4
, additive
R
R
R'
R
151
152
153
dr up to 99:1
ee up to 95 %
R=Ph,-CH
2
CH
2
Ph,
-CH
2
CH
2
CH
2
CH
3
,TMS
R' = -CH
2
CH
2
CH
2
CH
3
,cyclohexyl
Scheme 4.38
Highly diastereoselective Pauson-Khand reactions of the chiral propargylic
alcohols
151
.
4.4 Conclusion
In this chapter we disclose alternatives to the diastereoselective Pauson-Khand reaction for
the synthesis of various cyclopentenone derivatives. Both methodologies, the intra- and
intermolecular versions, seem very attractive and offer in some cases a great degree of
regioselectivity. In the majority of the examples, the transference of the chiral information
is high obtaining the valuable chiral cyclopentenones in excellent diastereoselectivities.