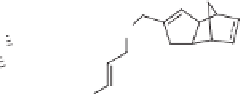Chemistry Reference
In-Depth Information
An interesting example of an intermolecular PK reaction of sugar-derived azaenynes was
described by Areces
et al.
in 2006.
37
Sugar azaenynes
137a,b
, which are readily obtained
fromD-glucal and D-galactal, respectively, react with norbornene and norbornadiene under
Pauson-Khand conditions to give intermolecular adducts
138
and
139
as the major or exclu-
sive product (Scheme 4.35). In all cases, only one diastereomer was obtained in a good yield
(up to 80%). This reaction represents an unprecedented example of a completely diastereos-
elective intermolecular Pauson-Khand reaction using a sugar moiety as the chiral auxiliary
TS
O
(i) Co2(CO)8/
CH2Cl2, RT, 45 min
.
(ii) NMO
N
Ts
N
Ts
N
or
H
H
+
H
H
O
R
R
R
137a-b
138a-b
139a-b
a) D-
erythro
b) D-
threo
R=(CHOAc)
2
CH
2
OAc
Scheme 4.35
Diastereoselective intermolecular Pauson-Khand reaction using a sugar moiety
as chiral auxiliary.
In 2010, Cazes
et al.
demonstrated that the PKR of allenic hydrocarbons
142
,gives
4-alkylidenecyclopentenones,
143
, with high regio- and stereo-selectivities (E/Z
70:30)
along with minor amounts of regioisomeric cyclopentenones
144
and
145
.
38
The regio- and
stereo-selectivities of isomeric cyclopentenones
143-145
depend mainly on the substitution
pattern of both the alkyne,
140
, and the allenic moieties,
142
(Scheme 4.36).
≥
R
3
(H)R
4
O
O
O
R
R
R
2
R
2
R
1
R
1
R
3
R
Co
2
(CO)
8
142
R
R
Co
2
(CO)
6
+
+
R
2
R
1
R
1
R'
R'
R'
R
3
R
3
R'
R'
R
2
140
141
143
144
145
Scheme 4.36
Intermolecular Pauson-Khand reactions of allenic hydrocarbons.
By studying the relationship between the change in selectivity of these isomeric cyclopen-
tenones,
143-145
, and the substitution patterns of both the acetylenic and allenic compo-
nents, competitive mechanistic pathways were established via several allene-dicobalt
complexes. DFT calculations were carried out on the most relevant of these intermediate
complexes; the results provided evidence for the first time that the PKR may involve both
pseudoequatorial and pseudoaxial coordination of a double bond to one of the cobalt atoms
leading to two isomeric cyclopentenones.
In the same year, Pu
et al.
developed catalytic asymmetric alkyne addition methodology
that provides rapid access to a variety of chiral propargylic alcohol-based enynes,
147,