Biomedical Engineering Reference
In-Depth Information
Figure 2.6
Continued
face of the helix, whereas hydrophobic amino acids line the other. The transmembrane sections
of polypeptides that span biological membranes often display one (or more)
-helical stretches. In
such instances, almost all the residues found in the helix display hydrophobic side chains.
β
α
-strands usually
are 5-10 amino acid residues in length, with the residues adopting an almost fully extended zigzag
conformation. Single
-strands represent the other major recurring structural element of proteins.
β
-strands are rarely, if ever, found alone. Instead, two or more of these strands
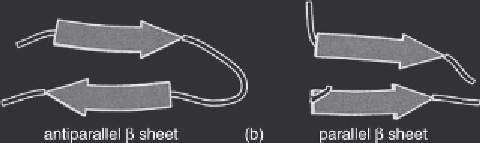
align themselves together to form a β-sheet. The β-sheet is a common structural element stabilized
by maximum hydrogen bonding (
Figure
2.6). The individual
β
-sheet
formation may all be present in the same polypeptide, or may be present in two polypeptides held in
close juxtaposition.
β
-strands participating in
β
-sheets are described as being parallel, antiparallel or mixed. A parallel sheet
is formed when all the participating β-stretches are running in the same direction (e.g. from the
amino terminus to the carboxy terminus;
Figure
2.6). An antiparallel sheet is formed when succes-
sive strands have alternating directions (N-terminus to C-terminus followed by C-terminus to N-ter-
minus, etc.). A
β
-sheet containing both parallel and antiparallel strands is termed a mixed sheet.
In terms of secondary structure, most proteins consist of several segments of α-helix and/ or
β
β
-strands separated from each other by various loop regions. These regions can vary in length and
shape, and allow the overall polypeptide to fold into a compact tertiary structure. In addition to
their obvious role in connecting stretches of regular secondary elements, loop regions themselves
often participate/contribute directly to the polypeptide's biological function. The antigen-binding
region of antibodies, for example, is largely constructed from six loop regions (Chapter 13). Such
loops also often form the active site of enzymes (Chapter 12). One loop structure, termed a β-turn
or
β
-bend, is a characteristic feature of many polypeptides (
Figure
2.7).
carbon of amino
acid residue 1
α
Hydrogen bond
C
1
2
O
H
β
turn
3
4
N
(a)
(b)
Figure 2.7
(a) The
β
-bend or
β
-turn is often found between two stretches of antiparallel
β
-strands. (b) It
is stabilized in part by hydrogen bonding between the C
=
O bond and the NH groups of the peptide bonds at
the neck of the turn


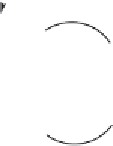





Search WWH ::

Custom Search