Biomedical Engineering Reference
In-Depth Information
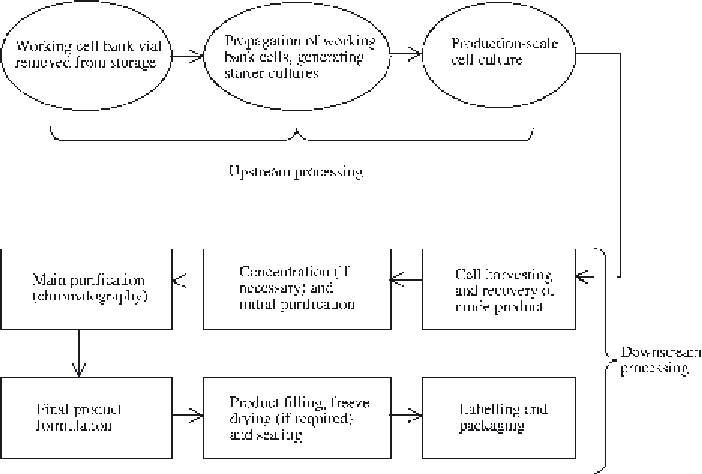
Figure 5.5
Overview of the production process for a biopharmaceutical product. Refer to text for specifi c
details
freeze-drying if a dried product format is required), followed by sealing of the fi nal product containers.
Subsequent labelling and packaging steps represent the fi nal steps of fi nished product manufacture.
Upstream processing is deemed to commence when a single vial of the working cell bank
system (see later) is taken from storage and the cells therein cultured in order to initiate the bio-
synthesis of a batch of product. The production process is deemed complete only when the fi nal
product is fi lled in its fi nal containers and those containers have been labelled and placed in their
fi nal product packaging.
5.3.1 Cellbankingsystems
Recombinant biopharmaceutical production cell lines are most often initially constructed by the intro-
duction into these cells of a plasmid housing a nucleotide sequence coding for the protein of interest
(Chapter 3). After culture, the resultant product-producing cell line is generally aliquoted into small
amounts, which are placed in ampoules and subsequently immersed in liquid nitrogen. Therefore, the
content of all the ampoules is identical, and the cells are effectively preserved for indefi nite periods when
stored under liquid nitrogen. This batch of cryopreserved ampoules forms a 'cell bank' system, whereby
one ampoule is thawed and the cell therein cultured in order to seed, for example, a single production
run. This concept is applied to both prokaryotic and eukaryotic biopharmaceutical-producing cells.
The cell bank's construction design is normally two tiered, consisting of a 'master cell bank' and a
'working cell bank' (
Figure
5.6). The master cell bank is constructed fi rst, directly from a culture of the
newly constructed production cell line. It can consist of several hundred individually stored ampoules.
Search WWH ::

Custom Search