Chemistry Reference
In-Depth Information
Other examples include the formation of the following.
(a) Complexes of the type H
2
[MBr
4
]
2−
upon reaction of cadmium, copper or lead with
hydrobromic acid:
(b) Complexes of the type H
2
M(CN
5
)
4
2−
upon reaction of cadmium, cobalt or zinc with
acidic ammonium thiocyanate:
(c) Formation of TlCl
4
3
− by reaction of thallous ion with hydrochloric acid.
High preconcentration factors can be achieved by such techniques, some further
examples of which are reviewed in Table 15.1.
15.1.7
Molybdate
Murthey and Ryan [63] used colloid flotation as a means of precon-centration prior to
neutron activation analysis for molybdenum. Hydrous iron(III) is floated in the presence
of sodium dodecyl sulphate with small nitrogen bubbles from 1L of sea water at pH 5.7.
Recoveries of molybdenum were better than 95%. This method has been used [64] to
precipitate from water samples traces of molybdenum. The trace element was
concentrated by coprecipitation with thionalide at pH 9.1. Co-precipitation with
thionalide allowed the concentration of both ions and colloids.
Molybdenum has been determined after preconcentration on Sephadex G.25 gel at pH
3.5. Ethylenediaminetetra acetic acid desorbs molyb-denum from the gel. This
preconcentration method brings the detection limit for molybdenum in fresh and sea
waters down to 1µg L
−1
in 250ml samples. The procedure was applied to 100ml solutions
containing 1.25µmol of molybdenum and 1- to 10000-fold amounts of various ions.
Interference from vanadium(V) and tungsten(VI) is probably due to complex formation
with molybdenum. Iron(III) at a 100-fold level lowered the recovery, probably because
its hydroxides or hydroxo complexes adsorb molybdenum, but the interference could be
overcome in the presence of 0.5mol L
−1
acetate. The method is suitable for the
determination of molybdenum at the levels normally encountered in river water (0.2-
0.6µg L
−1
) and is particularly effective for sea water.
Vasquez-Gonzalez
et al.
[65] have described a method for preconcen-trating and
determining molybdate by electrothermal atomisation atomic absorption spectrometry
after preconcentration by means of anion-exchange using Amberlite IRA-400 resin in
citrate form. The optimal analytical parameters were established by drying, carbonisation,
charring, atomisation and cleaning in a graphite furnace. The precision and accuracy of
the method were investigated. Less than 0.2µg L
−1
molybdate could be determined by
this procedure.
Fung and Dao [2] used Chelex-100 resin to remove molybdate from non saline waters
prior to desorption and determination at the µg L
−1
level.


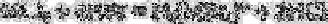

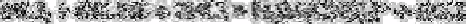
Search WWH ::

Custom Search