Chemistry Reference
In-Depth Information
15.1.5
Iodide and iodate
Iodide in non saline waters has been determined [24] by ion exchange high performance
liquid chromatography using an iodide ion selective electrode as detector. On-line
preconcentration of iodide on an anion guard cartridge allowed determinations down to
1nM.
Palagni [25] used the pulsed column bed technique to selectively separate and
preconcentrate low levels of iodide-127 in non saline water. The pulsed bed consisted of
a syringe containing a scintillation detector and a gamma-ray spectrometer. The
adsorbent consisted of open cell polyurethane foam impregnated with long chain tri-
n
-
alkyamine which formed a complex with iodide.
Carlsson
et al.
[5] preconcentrated bromide and iodide (and bromide), on an anion
exchanger, oxidised by peroxysulphate to bromate, treated with iodide, and measured the
absorbance of the resulting triiodide. The sum of bromate and iodate produced in the
oxidation was determined by treating the oxidised sample with iodide in hydrochloric
acid. The iodate was determined separately by applying the reaction in acetic acid. The
working range of the spectrophotometric method was 1-15µM, the limit of detection was
0.7µM for iodate and for iodate plus bromate and the enrichment factor in the
preconcentration was 50.
Iodide in non saline waters has been preconcentrated by transport extraction based on
solvent sublimation [26].
15.1.6
Metal cyanide complexes
Simple cationic metal species do not react with anion exchange resins. However, if the
metal ion M
+
is first reacted with a reagent with which it forms a negatively charged
anionic complex, then the resulting negatively charged metal-containing ions are retained
on an anion exchange resin. Thus, cadmium(II) forms a soluble anionic complex upon
reaction with potassium cyanide:
This complex is retained in a column of strong base anion exchange resin which, for
convenience, is represented as follows:
The cadmium complex can then be dissolved off the column with a small volume of
aqueous acetic acid to produce the acetate form of the resin and cadmium acetate:

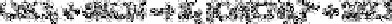

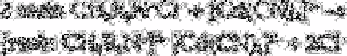


Search WWH ::

Custom Search