Chemistry Reference
In-Depth Information
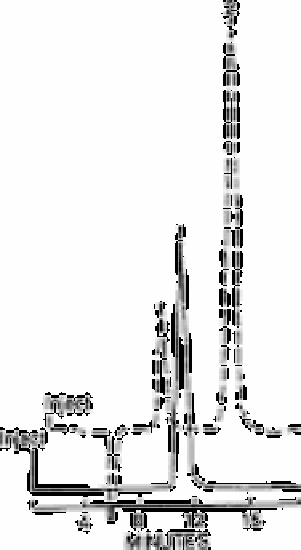
Fig. 13.5
Separation of arsenite and arsenate by ion exclusion chromatography;
conductivity detector (--); UV (- - -) at 200nm, position 1; peak 1,
15mg L−1 AsO
4
2−
; peak 2, 30mg L−1 AsO32−
Source: Own files
Ion exclusion, besides being a source of chromatographic interference, can also be used
to separate weak acids. Fig. 13.4 shows the separation of sulphide by ion exclusion
chromatography using a strong cation exchange column and water as the eluent. Peak 1 is
a combined peak resulting from both sulphite and sulphate, as monitored with the
conductivity detector. Peaks 2 and 3 correspond to sulphite and sulphide respectively, as
monitored using the ultraviolet detector. Since sulphide can be separated by either ion
chromatography or ion exclusion chromatography, the choice of separation mode will
depend on the sample matrix.
13.2.1.2
Arsenate and arsenite
Fig. 13.5 shows the separation of arsenite and arsenate by ion exclusion chromatography
using 0.01mol L
−1
hydrochloric acid as the eluent. Arsenious acid is a very weak acid and
cannot be detected at low levels by the conductivity detector. However, like sulphide, it is
easily detected with the ultraviolet detector. The simultaneous determination of arsenite
and arsenate is possible.
Arsenite can also be separated by ion chromatography using standard conditions. It
Search WWH ::

Custom Search