Chemistry Reference
In-Depth Information
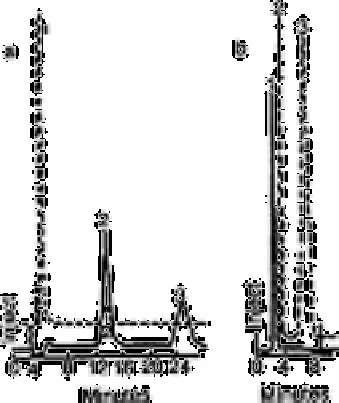
Fig. 13.4
Sulphide separator (a) ion chromatographic conditions conductivity
detector (--), UV detector (- - -) at 215nm position 1; peak 1, 10mg
L
−1
S
2−
; peak 2, SO
3
2−
; peak 3, SO
4
2−
; (b) ion exclusion
chromatographic conditions 4, conductivity detector (--), UV
detector (- - -) at 192nm; position 1; peak 1, SO
3
2−
and SO
4
2−
; peak
2, SO
3
2−
; peak 3, 15mg L−1
Source: Reproduced with permission from Elsevier Science [12]
with the conductivity detector while on only a minor 2% increase in peak height was
observed over the same time period by using the ultraviolet detector after the separator
column.
The ultraviolet detector can also be used in some cases to resolve overlapping peaks.
Determination of the nitrite peak by using the conductivity detector is complicated by
both the ion exclusion effect and the incomplete resolution between the large chloride
peak and the much smaller nitrite peak.
The ion exclusion interference can be eliminated for ultraviolet active anions by
placing the ultraviolet detector between the separator and suppressor columns (position
1). In addition, the problem of overlapping peaks can sometimes be resolved
spectrophotometrically by proper choice of wavelength.
Sulphide cannot be detected under normal ion chromatographic conditions. It is
converted into hydrogen sulphide in the suppressor column. Hydrogen sulphide acid is a
very weak acid that does not ionise sufficiently to be detected with the conductivity
detector. The ultraviolet detector, however, is able to detect sulphide at low levels, as is
illustrated in Fig. 13.4.
Since sulphide is a weak acid anion, the ultraviolet detector was placed between the
separator and suppressor columns. Fig. 13.4 also shows the presence of sulphite and
sulphate due to oxidation of the sulphide ion.
Search WWH ::

Custom Search