Chemistry Reference
In-Depth Information
Koupparis
et al.
[76] in which an average recovery of 98.6% was obtained for the
analysis of samples. Major interference is caused by reducing or oxidising ions. The low
results caused by copper(II) ion because of its catalysis of the decomposition of the
diazonium salt in methods with long reaction times are avoided in this method. The
serious sulphite interference is also almost eliminated in this method. The sulphide
interference can be eliminated by adding excess of cadmium ions and filtering. Addition
of 200mg L
−1
of Cd
2+
to a waste water sample containing 1mg L
−1
of NO
2
−
-N and
50mg L
−1
of sulphide eliminated the error.
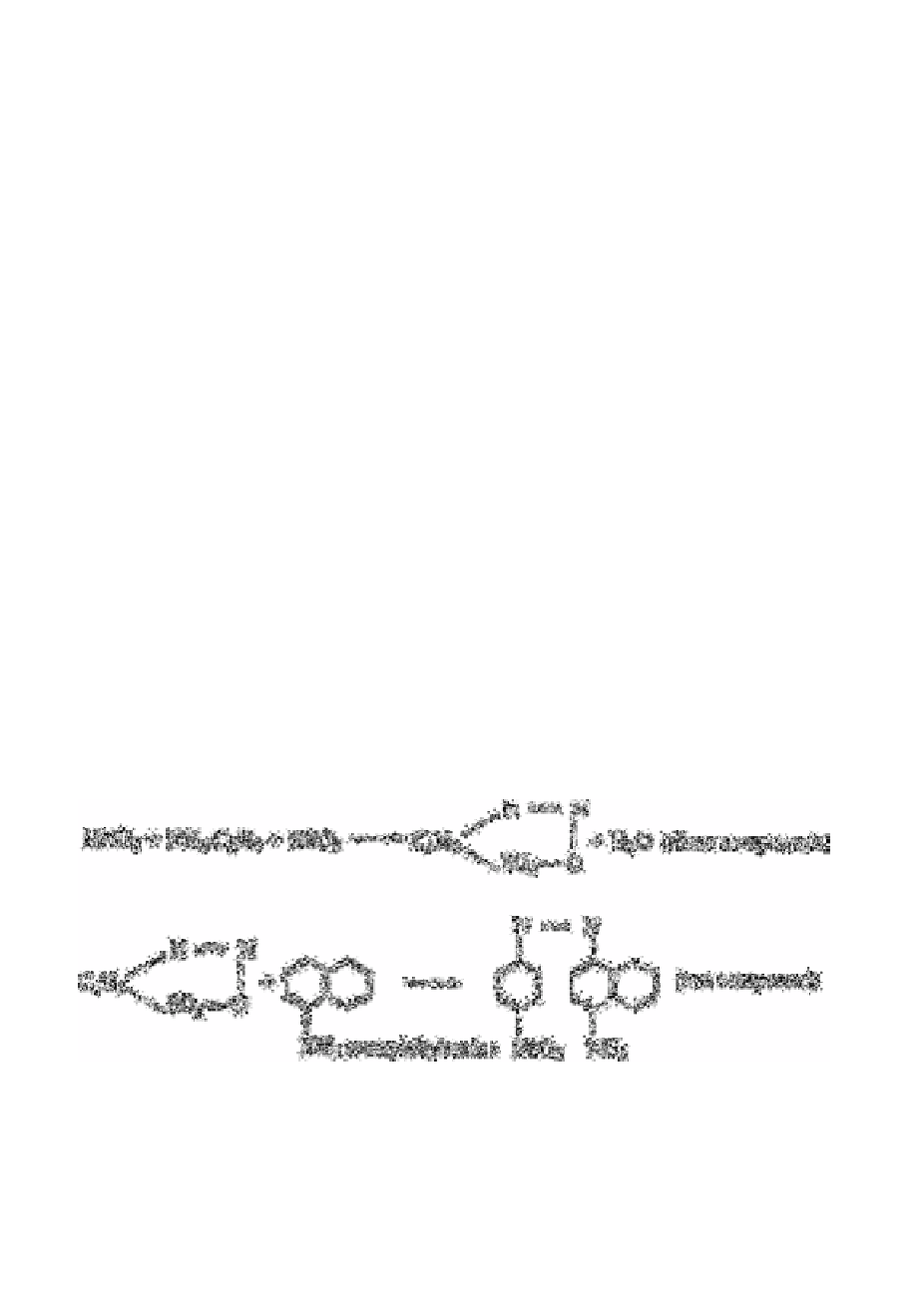
Various other workers [77-79] have discussed the determination of nitrite by methods
involving the formation if a chromophore with an absorption maximum at 540nm
following the formation of a red-purple diazo complex by reaction with sulphanilamide
and coupling with
N
-(1-naphthyl)ethylene diamine. The application of this technique is
also discussed under multianion analysis in section 14.2.3.1.
8.15.2
Electrophoresis
The application of this technique is discussed under multianion analysis in section 7.28.1.
8.15.3
Roman spectroscopy
Furaya
et al.
[71] have developed a method for the determination of nitrite in amounts
down to 0.5µg L
−1
in waste and treated waters by resonance Raman spectrometry after
conversion of nitrite to coloured azo dyes. Nitrate can also be determined simultaneously
by this method. Due to the resonance effect, the intensities of spectra often become five
or six orders of magnitude larger than those of usual Raman spectra. Therefore, Furaya
et
al.
[71] attempted to determine nitrite by use of resonance Raman spectrometry.
This method, however, can be applied only to coloured compounds and aqueous
solutions of nitrite are completely colourless. Therefore, nitrite was converted to a
coloured product by diazotisation and after that the resonance Raman spectrum was
measured.
Search WWH ::

Custom Search