Chemistry Reference
In-Depth Information
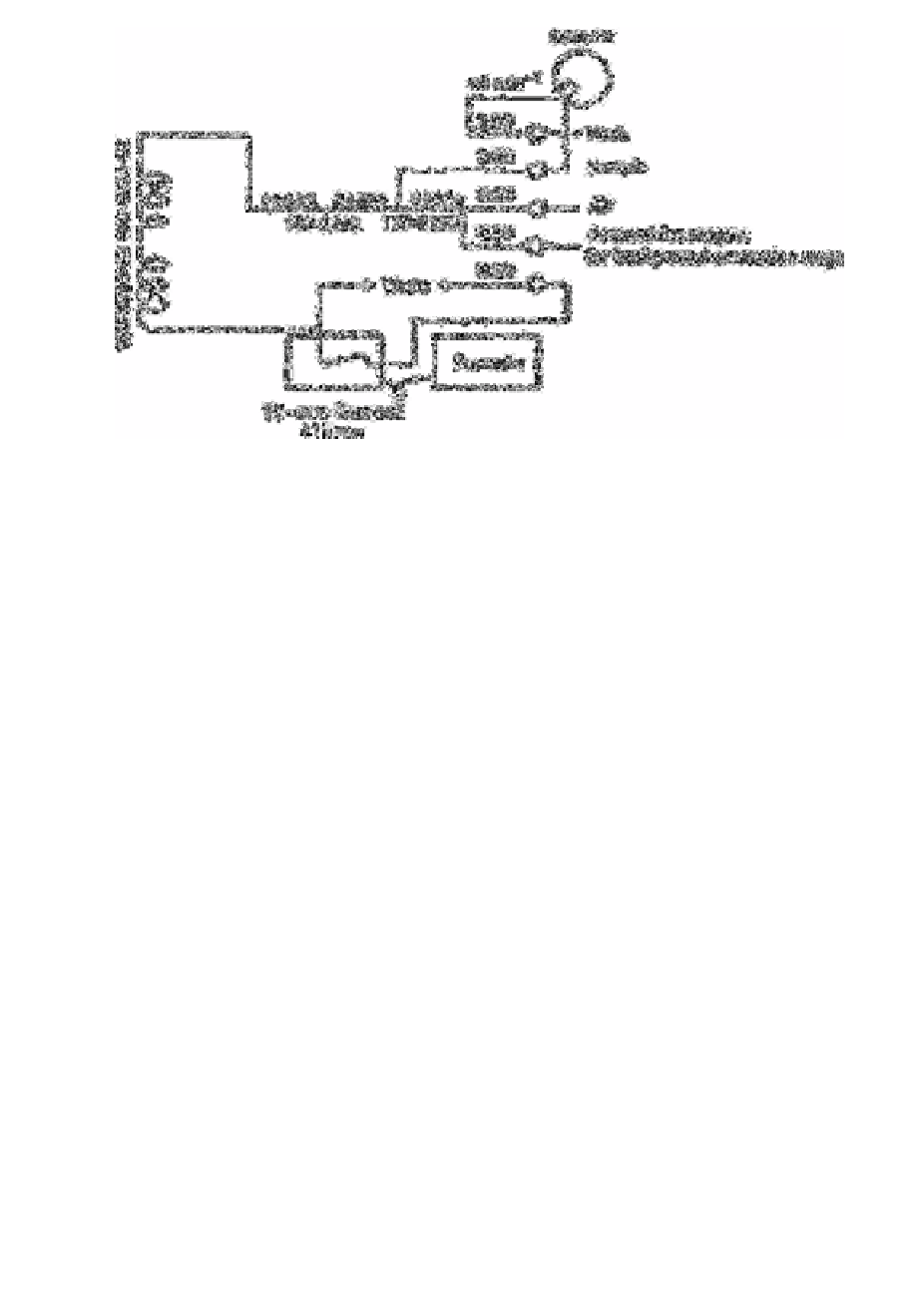
Fig. 8.1
Manifold arrangement for AutoAnalyzer 2
Source: Reproduced with permission from the Royal Society of
Chemistry [7]
The curcumin procedure does not involve these dangers, but includes an evaporation
stage unsuitable for automation. A method employing ferroin has been developed [6]
involving extraction into chloroform but this method suffers from interference by anionic
detergents.
Edwards [7] has developed a method employing azomethine-H (a condensation
product of H-acid, (8-aminonaphthyl-1-ol-3, 6 disulphonic acid and salicylaldehyde) in
aqueous medium, for the determination of boron in raw waters and effluents. This reagent
is very sensitive to borates, forming, in aqueous medium, a yellow ion associated
compound by reversible reaction. The method is capable of a limit of detection of 0.1mg
L
−1
and the response is essentially linear to 4.0mg L
−1
, although a calibration graph is
required above this level. A wide range of possible interferences was tested, but none
proved of practical importance except sample colour, for which adequate correction is
provided. Recovery after spiking a typical range of raw and waste waters was adequate.
A Technicon Auto Analyser 2 system is fitted with the manifold shown in Fig. 8.1. A
sample cam of 30h−1 is employed, with a 2:1 sample to wash water ratio; 410nm
wavelength filters are fitted.
The Azomethine-H reagent is prepared as follows: dissolve 18g of H-acid in 1L of
water with gentle heating, neutralise to pH 7.5 ±0.5 with 10% w/v potassium hydroxide
solution and filter if necessary. Add concentrated hydrochloric acid, to pH 1.5 ±0.1, while
still warm. Add 20ml of salicylaldehyde and stir vigorously while heating gently (not
Search WWH ::

Custom Search