Chemistry Reference
In-Depth Information
Chapter 8
Anions in wastewaters
8.1
Borate
The level of boron in raw waters has tended to increase, resulting from its greater use in
cleansing materials and in industrial processes [1]. As sewage treatment does not
significantly reduce this level the increase is passed to surface waters, which may then be
utilised for crop irrigation or potable water supply. Many varieties of fruit can tolerate no
more than 0.5mg L
−1
of boron [2] in irrigation water. The US Environmental Protection
Agency [3] recommends limits of 0.75mg L
−1
for most fruits, 1.0mg L
−1
for most cereals,
potatoes, peas and tomatoes and 2.0mg L
−1
for tolerant species including sugar beet,
turnips and cabbage. The Anglian Water Authority [4] apply criteria such that the
'maximum desirable
'
limit for crop irrigation is 0.5mg L
−1
and the 'maximum
permissible' limit is 1.0mg L
−1
. They also apply criteria of 0.8 and 1.0-1.2mg L
−1
,
respectively, to potable water abstractions.
8.1.1
Spectrophotometric method
Several reagents have been developed for the routine determination of boron, but most
require a final stage in concentrated sulphuric acid. Apart from the hazards involved in
pumping the concentrated acid the procedures are difficult to automate, principally
because of density effects resulting from small changes in acid concentration. These
methods include the carminic acid procedure, which has been automated by the Water
Pollution Research Laboratory [5]. This method is not very precise.

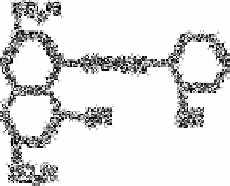
Search WWH ::

Custom Search