Chemistry Reference
In-Depth Information
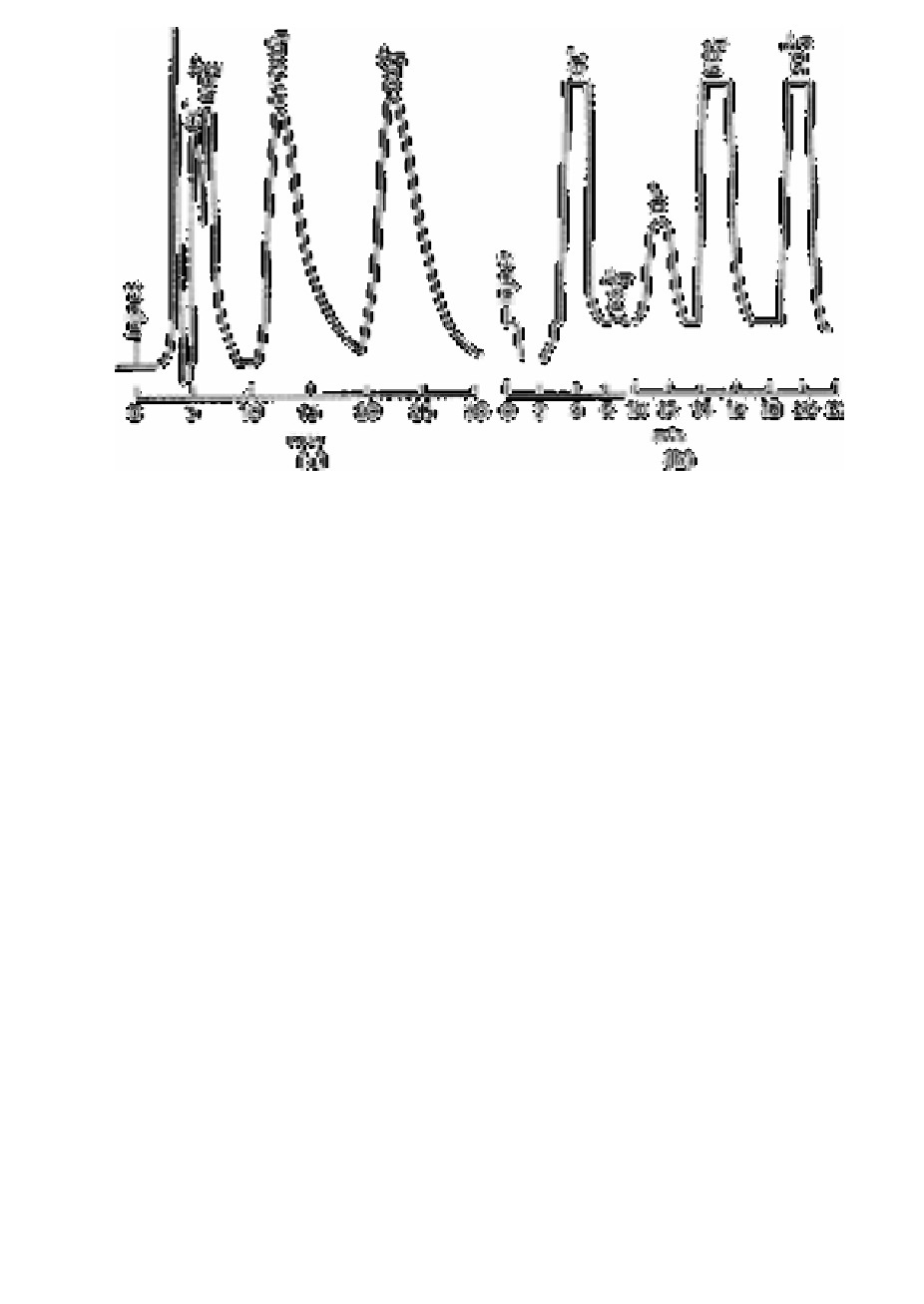
Fig. 7.6
Ion chromatography on anions in potable water, (a) Simultaneous
elution of bromide and nitrate using standard eluant; (b) separation of
bromide and nitrate using trace anion separator and standard eluant
Source: Reproduced with permission from Elsevier Science [10]
ion. There was no response for 80µg L
−1
bromide in the presence of 1000mg L
−1
sulphate, indicating 0% recovery.
Nitrate in excess of 17mg L
−1
interfered with the elution of bromide and sulphate in
excess of 25mg L
−1
interfered with the elution of bromide.
The application of this technique is discussed under multianion analysis in sections
12.6.2 and 12.6.3.
7.6.3
High performance liquid chromatography
Morrow and Minear [10] have described an ion chromatographic method for the
determination of bromide in potable water.
The application of this technique is discussed under multianion analysis in section
13.1.2.1.
7.6.4
Gas chromatography
Nota
et al.
[11] have devised a simple and sensitive method for the determination of
amounts of bromide down to 0.05mg L
−1
in water. The procedure is in two stages.
Cyanogen bromide is formed by the reaction between bromide, chlorine and cyanide,
Search WWH ::

Custom Search